Abstract
Pancreatic cancer is an aggressive cancer with a dismal prognosis. This is due to the difficulty to detect the disease at an early and curable stage. In addition, only limited treatment options are available, and they are confronted by mechanisms of resistance. Monoclonal antibody (mAb) molecules are highly specific biologics that can be directly used as a blocking agent or modified to deliver a drug payload depending on the desired outcome. They are widely used to target extracellular proteins, but they can also be employed to inhibit intracellular proteins, such as oncoproteins. While mAbs are a class of therapeutics that have been successfully employed to treat many cancers, they have shown only limited efficacy in pancreatic cancer as a monotherapy so far. In this review, we will discuss the challenges, opportunities and hopes to use mAbs for pancreatic cancer treatment, diagnostics and imagery.
Keywords: pancreatic cancer, monoclonal antibody, antibody drug conjugate, imaging, intracellular antibody, KRAS, chemotherapy
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common malignancy of the pancreas (here also referred to as pancreatic cancer). It is a devastating disease with a 5-year overall survival of 11% and is predicted to be the second largest cause of cancer death by 2040 [1]. Several reasons can explain such a poor prognosis: it is usually diagnosed at a late stage, which is often due to non-specific symptoms, a lack of sensitive and specific tumour markers and difficulties in imaging early-stage tumours. In addition, a lack of effective therapies due to the resistance to treatments, such as chemotherapy or radiotherapy, is observed in PDAC. The only potentially curative solution for PDAC patients is surgery, but less than 20% of patients can benefit from a resection [2]. Depending on the stage of the disease, different chemotherapies can be used, including the standard of care for PDAC patients, gemcitabine, a nucleoside analogue approved in 1997 [3]. FOLFIRINOX is a combination of folinic acid (FOL), 5-fluorouracil or 5-FU (F), irinotecan (IRIN) and oxaliplatin (OX) and was approved in 2011 by the Food and Drug Administration (FDA) [4]. Nab-paclitaxel is an albumin-bound nanoformulation of paclitaxel (a taxol derivative) that was approved in 2013 by the FDA in combination with gemcitabine [5]. FOLFIRINOX and nab-paclitaxel (combined with gemcitabine) are mostly used in advanced PDAC. At the molecular level, PDAC is a complex cancer that harbours multiple genetic and epigenetic alterations, making this cancer highly heterogeneous. Based on transcriptomic analyses, PDAC tumours and stroma have been each divided into two predominant molecular subtypes: classical and basal-like for the tumour tissue, normal and activated for the stromal tissue to help stratify patients and find specific therapeutic strategies [6]. However, recent studies showed that PDAC tumours and their microenvironment are more complex and can be classified into several categories [7]. Therefore, understanding the molecular features and the immune landscape of PDAC is essential to develop novel efficient (targeted) therapies. This is exemplified with the FDA approval of PARP inhibitor olaparib for patients with germline BRCA1 or BRCA2 mutations in platinum-sensitive metastatic PDAC [8], further demonstrating the importance of deciphering the tumours at the molecular level. However, multiple hurdles need to be overcome in PDAC to improve patients’ outcomes, such as fighting therapeutic resistance, discovering early detection methods and effective therapeutics.
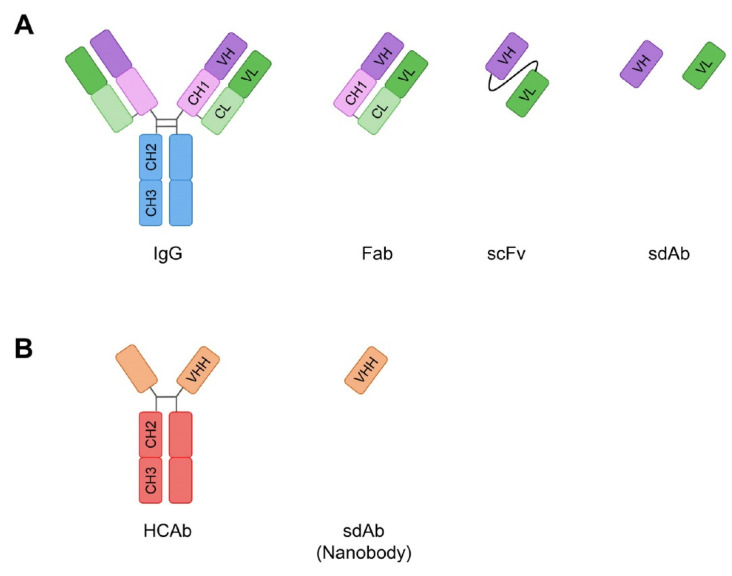
Monoclonal antibodies (mAbs) are an attractive avenue to answer these challenges in PDAC. They exist in different formats, such as full-length immunoglobuline (Ig) or as antibody fragments (e.g., fragment antigen binding (Fab), single chain fragment variable (scFv), single domain antibodies) (Figure 1). Each of them has advantages/drawbacks depending on their final application. For instance, larger antibody formats (i.e., full size or Fab) will usually have a longer half-life in the bloodstream compared to smaller fragments (scFv or single domains), but the latter could penetrate into tumours more easily due to their small size. Antibodies are versatile molecules that can be selected in vitro by phage display and used as direct blocking therapeutics for intracellular or extracellular proteins [9,10]. When conjugated, they can deliver a drug payload, such as antibody drug conjugate (ADC) [11] or radiolabelled antibodies, for targeted radionuclide therapy [12]. Finally, if functionalised with a fluorescent or radio-analogue moiety, mAbs can be used as an imaging agent.
Figure 1.
Different antibody formats. (A) Full-length immunoglobulin G (IgG) and its derivatives Fab (fragment antigen-binding), scFv (single chain fragment variable) and sdAb (single domain antibody). VH (variable heavy) and VL (variable light) are the domains that enable the binding to the antigen. (B) Heavy chain only antibody (HCAb) found in Camelidae and its derivative VHH (variable heavy domain of heavy chain only antibody) that is a sdAb and is also called nanobody.
Here, we review the potential of antibodies to target cell surface or intracellular proteins in PDAC but also how they can be modified to kill cancer cells or specifically detect tumours.
2. Targeting the Surfaceome of Pancreatic Tumours
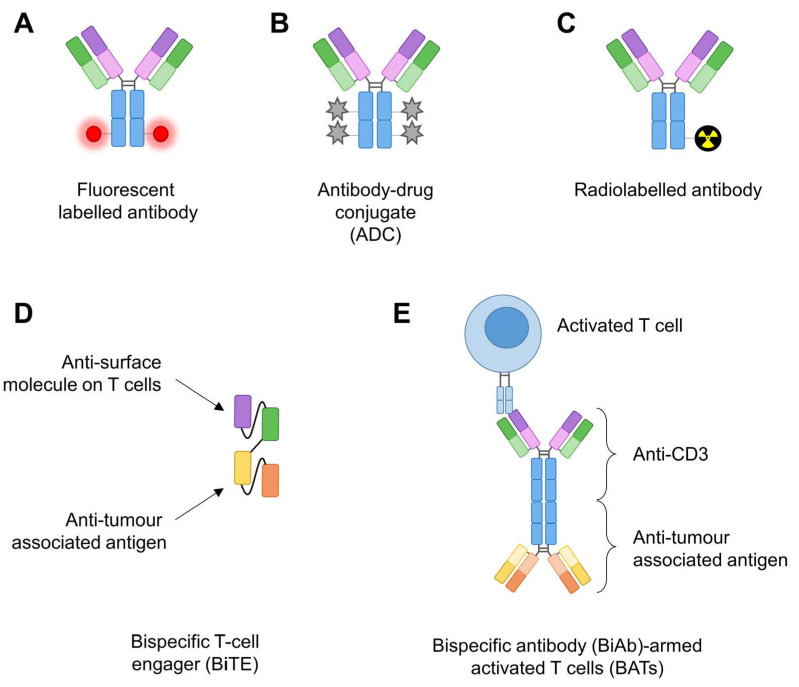
Around 25–30% of human genes encode for cell surface proteins, also called surfaceome [13]. Some membrane-bound proteins have been successfully targeted by antibodies to directly block downstream signalling pathways or, when functionalised with molecules, to image tumours for diagnostic or surgery or to specifically kill cancer cells (e.g., ADC or targeted radionuclid therapy) (Figure 2).
Figure 2.
Antibodies’ functionalisation for cancer therapy. (A) Fluorescent labelled antibody: a fluorescent dye (usually far-red) is linked to an antibody. (B) Antibody-drug conjugate (ADC) is an antibody modified with cytotoxic drugs. (C) Radiolabelled antibody is linked to a radioisotope moiety for targeted radionuclid therapy and/or imaging. (D) Bispecific T cell engager (BiTE) is usually two scFvs linked together: one binding a tumour-associated antigen (TAA) and the other one a surface molecule on T cells. (E) Bispecific antibody-armed activated T cells (BATs) correspond to two full-length IgGs crosslinked and incubated with autologous activated T cells (i.e., from patients), and the BATs are reinfused back into the patients.
2.1. EGFR
EGFR belongs to the family of transmembrane tyrosine kinase receptors that includes HER receptors. It is mutated and also overexpressed in numerous cancers, including pancreatic cancer, making this receptor a key therapeutic target. EGFR-mediated signalling plays a role in proliferation, metastasis and apoptosis evasion [14]. Therapeutic targeting of EGFR by erlotinib, a tyrosine kinase inhibitor, combined with gemcitabine has shown modest but reproducible responses in patients with unresectable metastatic pancreatic cancer, leading to its approval by the FDA [15]. Several preclinical studies using pancreatic cancer xenograft in nude mice have supported the strategy to disrupt EGFR-mediated signalling with cetuximab [16], a monoclonal chimeric IgG1 that targets the receptor protein expressed on the cell surface [17]. In addition, combination of gemcitabine and cetuximab in an orthotopic PDAC mouse model showed an additive anti-tumour effect [18]. These studies, added to the success of cetuximab in colorectal cancer, prompted the investigation of anti-EGFR therapies in PDAC patients. However, the phase III study results were disappointing and showed that gemcitabine plus cetuximab treatment did not improve the patients’ outcomes compared with patients treated with gemcitabine alone [19]. Furthermore, patients in the cetuximab/gemcitabine arm experienced more frequently grade 4–5 toxicities compared to the gemcitabine arm [19].
While targeting EGFR with cetuximab and chemotherapy was not successful, the anti-EGFR therapies focus is now on repurposing their use in pancreatic cancer. Consequently, anti-EGFR antibodies have been functionalised with molecules to either monitor, target or kill, more specifically, pancreatic tumours. Near-infrared (NIR) fluorescence is a promising technology to help visualise the tumour during surgery. Anti-EGFR antibody panitumumab was linked to a fluorescent fluorophore (IRDye800CW, Figure 2A), and a clinical trial was performed in patients with pancreatic cancer undergoing surgery (NCT03384238). This study showed this fluorescent tracer is safe and feasible to use during PDAC surgery [20]. A humanised anti-EGFR antibody-based ADC (Figure 2B) was developed by conjugating monomethyl auristatin E (MMAE), a microtubule destabiliser, to the antibody. This ADC is specific to EGFR expressing cells, is only cytotoxic in these cells in vitro and showed anti-tumour activity in vivo [21], but it is still not tested in clinical trials. Cetuximab was used as a targeting agent for camptothecin (CPT, a chemotherapy) encapsulated into polymeric nanoparticules. Cetuximab nanoconjugation enhanced the CPT delivery in vitro and improved the growth-inhibitory effects in vivo [22]. Cetuximab was also conjugated to murine IgG2a anti-CD3 mAb (OKT3) to make a bispecific antibody. Activated T cells were armed with this bispecific Ab (EGFR BATs, Figure 2E) to enhance receptor-directed cytotoxicity [23]. EGFR BATs are ex-vivo-expanded autologous activated T cells armed with a bispecific Ab that are reinfused back into the patient. These engineered T cells showed an anti-tumour effect in preclinical settings [23], and the first results of two clinical trials involving seven patients (NCT01420874, completed phase Ib; NCT02620865, completed phase II) are encouraging. Actually, infusions of BATs in patients are safe (only toxicities grade 1–3 side effects and no dose-limiting toxicities), induced anti-pancreatic cancer cytotoxicity with immune and cytokine responses and a median overall survival (OS) of 31 months for the seven patients [24]. These promising results will need to be confirmed with a larger cohort of patients.
Even though EGFR antibodies showed no benefit to treat PDAC patients, a study by Blasco and collaborators showed that genetic removal of EGFR and CRAF kinase in a mouse model induced the regression of PDAC tumours in vivo, suggesting that a combination of EGFR and CRAF inhibitors could be of interest for PDAC treatment [25], but this has still to be assessed.
2.2. Mesothelin
Mesothelin (MSLN) is a 40 kDa glycosyl phosphatidylinositol anchored cell surface protein expressed on mesothelial cells. The physiological role of MSLN remains unknown, but it seems non-essential because mice harbouring a null mutation in the mesothelin gene exhibit normal development and reproductive capabilities [26]. Interestingly, normal pancreatic tissue does not express RNA coding for the precursor of mesothelin nor mesothelin protein, but it is overexpressed in several cancers, including PDAC [27]. In addition, overexpression of MSLN in PDAC is found in almost all tumours, which is interesting knowing the particularly high heterogeneity of these tumours. All those characteristics point towards MSLN as a target of interest in PDAC.
Hence, a recombinant anti-mesothelin immunotoxin, named SS1P, was developed by fusing a mouse anti-MSLN disulfide-stabilized Fv antibody fragment (dsFv, named SS1) to a truncated fragment of Pseudomonas exotoxin A (PE) [28]. The preclinical data using this antibody-based therapy were promising as combination of treatment of SS1P with radiation, taxol or gemcitabine resulted in enhanced antitumor activity against mesothelin-expressing tumour xenografts [29,30,31]. Then, a phase I clinical trial that included two PDAC patients was conducted by treating patients with SS1P, but the results showed modest clinical activity. Actually, 88% of the patients developed neutralising antibodies against SS1P due to the high immunogenicity of PE, thus limiting the treatment to only one cycle of therapy [32]. To overcome this immunogenicity in patients, Mossoba et al. showed, in a proof-of-concept preclinical study, that an immune depletion regimen could abrogate anti-immunotoxin reactivity [33]. Therefore, a phase II study that consisted of SS1P treatment combined with pentostatin and cyclophosphamide, two drugs that help suppress the immune system, was initiated. Unfortunately, around 20% of the patients still developed neutralising antibodies and none of the PDAC patients completed the trial because of adverse events or progressive disease (NCT01362790). After the failure of SS1P, a second generation of immunotoxin was designed with a low-immunogenic modified PE fragment fused to a humanised anti-mesothelin Fab fragment and named RG7787 and later on LMB-100 [34]. Preclinical studies of RG7787 in combination with paclitaxel in pancreatic cancer showed durable anti-tumour responses [34]. However, it is worth noting that this immunotoxin could only reach 45% of the tumour in vivo [34], highlighting the barrier role of the PDAC dense stroma. A follow-up study showed the synergic effect in a PDAC mouse model treated with LMB-100 and nab-paclitaxel with complete regression of tumours treated by this combination [35]. These data led to a clinical trial of LMB-100 combined with nab-paclitaxel on patients with advanced PDAC (NCT02810418). Although clinical activity was observed, the combination was not well tolerated by patients [36]. Anetumab ravtansine, an ADC of anti-MSLN antibody linked to maytansinoid DM4 drug, was tested in a phase I study in metastatic solid tumours, including pancreatic cancer (NCT01439152) [37]. Anetumab ravtansine showed manageable safety with encouraging anti-tumour activity [37]. A phase II study followed and is completed, but the data are pending (NCT03023722).
Amatuximab or MORAb-009 is a chimeric anti-mesothelin Ab that was developed by grafting the mouse VH and VL fragments of SS1 with human IgG1 and kappa constant regions. This Ab elicited antibody-dependent cellular cytotoxicity (ADCC) on pancreatic cancer cells in vitro and blocked the interaction between MSLN and its ligand CA125/MUC16 [38]. Interestingly, this interaction is thought to facilitate metastasis of cancer cells [39]. Preclinical studies showed promising results by enhancing the anti-tumour effects of gemcitabine and taxol in vivo [38,40]. The results of phase I studies revealed that MORAb-009 is well tolerated [41] and led to phase II studies with MORAb-009 in combination with gemcitabine (NCT00570713). However, this trial was not completed because of a lack of efficacy in more than 50% of the patients (no improvement in OS or progression-free survival (PFS) compared to the control group gemcitabine only). Moreover, amatuximab was tested alone in another clinical trial (NCT01413451) on patients with cancers expressing high levels of mesothelin (including PDAC patients). Even though no results were published, a differential biodistribution of MORAb-009 uptake level was shown that is higher in mesothelioma patients compared to PDAC patients using single photon emission computed tomography-computed tomography (SPECT-CT) imaging [42]. This echoes to other studies discussed above and highlights again the issue for biologics to go through the dense tumoral microenvironment (TME) of PDAC tumours. Overall, these studies show how difficult it is to translate encouraging preclinical data into the clinic. Nevertheless, functionalising anti-MSLN mAb as ADC [37] or using anti-MSLN scFv in a chimeric antigen receptor T cell (CAR T cell, reviewed in Ref. [43]) therapy [44,45] might be a solution to achieve efficacy in patients.
2.3. Mucins
Mucins are a family of multifunctional glycoproteins expressed on the surface of epithelial cells in the gastrointestinal tract that are playing pivotal roles in gut lubrication and protection. Almost all proteins among this family are globally overexpressed in pancreatic cancer [46]. Actually, they form a protective coat around cancer cells and have important roles in the carcinogenesis of PDAC [47]. They are involved in many malignant processes, including evasion, invasion and metastasis, by affecting oncogenic signalling, cell survival, proliferation and resistance to chemotherapeutics [48]. Moreover, mucins in PDAC present a specific pattern of expression during the different steps of tumour progression toward carcinoma [46]. This family of proteins, and particularly MUC1/4/5, have drawn attention and investigation, such as new biomarkers and therapeutic targets in PDAC, notably with antibody-based therapy [49].
Gatipotuzumab, also known as PankoMab-GEX, is a humanized IgG1 anti-MUC1 mAb that binds with high affinity to a novel carbohydrate-induced conformational epitope on MUC1 (named tumour-related MUC1 epitope, TA-MUC1) [50]. TA-MUC1 is highly expressed in a broad variety of carcinomas and virtually not expressed on normal cells. This mAb is, therefore, displaying numerous advantages compared to other anti-MUC1 antibodies in clinical development with higher tumour specificity, higher affinity and rapid internalisation [50]. Hence, Gatipotuzumab was tested in phase I clinical studies on patients with advanced carcinomas, including PDAC (NCT01222624). The data revealed that the mAb is safe, well tolerated, and showed promising anti-tumour activity in advanced disease. Of note, adverse events were mainly mild-to-moderate infusion-related reactions in about 50% of the patients. However, there was no efficacy on pancreatic cancer patients [51], and this mAb is still not approved for clinical use. Because Gatipotuzumab internalises rapidly in cancer cells, this property prompted the companies Glycotope and Daiichi Sankyo to develop an ADC version of this mAb that is currently under preclinical assessment [52].
Another anti-MUC1 mAb was developed, PAM4, that shows high specificity for MUC1 expressed by PDAC compared to other cancers, normal pancreas or pancreatitis [53]. Taking advantage of its high specificity, the humanised version of this mAb was radiolabelled (Figure 2C) and used for either nuclear imaging when PAM4 was labelled with an indium 111 (111In) radioisotope or for targeted radionuclid therapy when PAM4 was functionalised with yttrium-90 (90Y) radioisotope [54]. 90Y-PAM4 showed anti-tumour response with reasonable adverse effects in vivo [55]. Therefore, these results prompted the use of humanised PAM4 antibody labelled with the aforementioned radioisotopes in an early phase I clinical study in patients with PDAC (NCT00364364). Unfortunately, this clinical trial was terminated without any data due to loss of funding.
PAM4 mAb is selective of MUC1 expressed by PDAC; hence, its ability to detect the early stage of PDAC was tested by immunohistochemistry. While another anti-MUC1 antibody, MA5, stained several normal tissues (e.g., pancreas, colon, stomach, lung) and many tumour tissues, PAM4 did not, confirming its PDAC specificity. In addition, PAM4 labelled 94% of the earliest PanIN lesions (PanIN-1A, -1B) by immunohistochemistry [56], showing promising results to employ this mAb for the detection of early-stage disease. Finally, an anti-TA-MUC1 mAb (5E5) was developed [57] and employed successfully in a CAR T cell therapy setting in a preclinical study [58].
MUC4 is not expressed in healthy pancreas, but it is overexpressed in 70–80% of pancreatic cancer [59]. Interestingly, MUC4 expression is linked to resistance to treatment in PDAC, including gemcitabine, the standard of care for PDAC patients [60]. Hence, targeting MUC4 could be useful for tumour detection and therapy, but the development of therapeutic mAbs is required. MUC5AC is also overexpressed in PDAC, with minimal expression in healthy pancreatic tissue, which makes it a targetable marker in this cancer [61]. Therefore, several groups used or developed anti-MUC5AC mAbs for therapy or tumour detection. When linked to 111In [62] or 89Zirconium (Zr) [63] radioisotopes or to a NIR dye (IRDye800CW) [64], anti-MUC5AC mAbs could preferentially target pancreatic cancer tissue compared to normal pancreatic tissue. In the future, fluorescent mAbs could help guide the resection of PDAC. A humanised anti-MUC5AC antibody was labelled with 225Actinium (Ac) for targeted radionuclide therapy and demonstrated efficacy to suppress tumour growth in mice [63]. However, none of these MUC5AC antibodies are currently in clinical trials.
2.4. CEA
Carcinoembryonic antigen (CEA), or CEACAM-5, is a cell adhesion molecule anchored to the cell membrane involved in extracellular matrix adhesion, motility and inhibition of apoptosis [65]. It plays a crucial role in a number of biological processes, including homeostasis, embryogenesis and development of neural tissue, inflammation, immune cell transmigration and immune response. However, CEA is not expressed in healthy pancreas and moderately expressed in pancreatitis, strengthening its potential as a PDAC-specific target [66]. After carbohydrate antigen CA19-9, CEA is the second most used biomarker for diagnosis and monitoring of PDAC. As a matter of fact, CEA serum levels are increased in 40–70% of all PDAC patients, while CEA is overexpressed on the cell membrane in 70–85% of PDAC cases [67]. Recently, a retrospective analysis showed that CEA before neoadjuvant chemoradiotherapy is a crucial prognostic indicator for localised PDAC [68]. Therefore, mAbs were developed to target CEA for image-guided surgery to detect and/or inhibit PDAC tumours but also as anti-CEA CAR T cell therapy (NCT03818165). However, this clinical trial was terminated as a consequence of a limited number of recruited patients. A chimeric fluorescent ADC anti-CEA mouse antibody was tested for its ability to detect and inhibit tumour growth in vivo [69]. This mAb was linked to paclitaxel and an infrared fluorophore (DyeLight680) and showed the feasibility to (i) localise the tumour with long-lasting effect and to (ii) impede tumour growth in vivo [69]. Nevertheless, it is important to note that paclitaxel is not a drug approved for PDAC therapy and that the mouse origin of the antibody could limit its direct use in patients. Therefore, additional optimisation steps of this fluorescent ADC are required before a potential clinical application. However, an ADC anti-CEA humanised mAb (tusamitamab ravtansine) is currently under clinical trial for patients with metastatic PDAC and breast cancer (phase II, NCT04659603). The same mAb was designed as a fluorescent molecule when linked to IRDye800CW and showed promising results to probe human pancreatic cancer in vivo with a favourable tumour-to-background ratio [70].
So far, the only fluorescent mAb that is in clinical trial is SGM-101, an anti-CEA chimeric mAb that is engineered with an original NIR dye (BM104). This fluorescent tracer specifically labelled the tumours from an orthotopic pancreatic cancer model in vivo using BxPC-3 cells with a tumour-to-background ratio of 3.5 [71]. Based on these encouraging results, a phase I clinical trial was performed (NCT02973672) and showed the use of SGM-101 is safe (no adverse events were observed in patients except one with diarrhoea) and feasible for the detection of both primary PDAC and metastasis [72]. While SGM-101 is now tested in several phase II clinical studies for colorectal cancer metastasis (NCT04737213, NCT03659448, NCT04755920), it is still not the case for pancreatic cancer. This is probably because additional prospective research is needed to test whether this technique will ultimately improve OS of PDAC patients. Moreover, image-guided surgery of PDAC tumours could also require the use of other agents targeting the tumour stroma, abundant in pancreatic cancer, to increase sensitivity [72]. Full-length mAbs are useful for image-guided surgery because of their long half-life fluorescence signal in the tumour. However, it takes 2 to 3 days to obtain the tumour fluorescence because the accessibility of the tumour by the mAb is limited by the antibody’s size. Hence, developing smaller antibody fragments, such as sdAbs (e.g., nanobodies), could circumvent this drawback. Recently, an anti-CEA nanobody conjugated to an IR800CW dye was developed to target and label patient-derived pancreatic cancer xenografts in mice. It efficiently reached the tumour within an hour with a good tumour-to-background ratio (of 2.0 by 3 h) and allowed a durable fluorescence signal over hours. These characteristics make this fluorescent nanobody a promising and practical molecule for precise fluorescence-guided surgery of pancreatic cancer [73]. Interestingly, if the anti-CEA nanobody targets another epitope than the mAb, a combination of both fluorescent mAb and nanobody could enable a more rapid fluorescence of the tumour within hours to accelerate the patients’ care with a durable fluorescence signal useful for long surgery, such as pancreatic cancer resection.
2.5. Exploring the Surfaceome for the Discovery of Novel Targets and Biomarkers
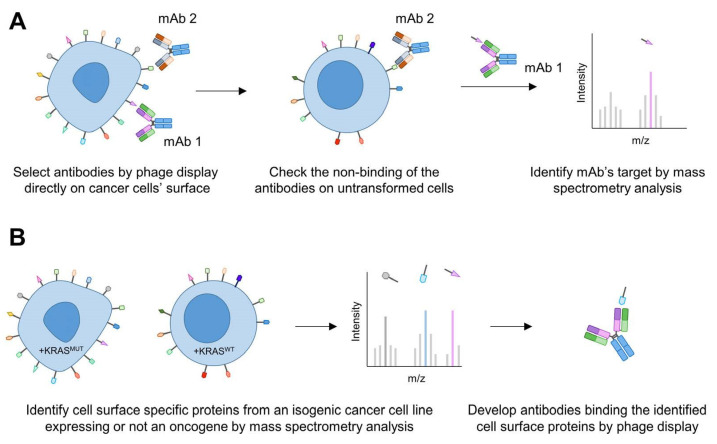
As discussed above, only a few membrane proteins have been targeted in pancreatic cancer; therefore, the discovery of novel (pancreatic) cancer-specific targets is necessary. To this end, two different methods have been implemented for the development of membrane-protein-specific antibodies (Figure 3).
Figure 3.
Discovery of novel cell surface proteins with antibodies. (A) Selection of mAbs directly binding to cancer cells by phage display and that do not bind to untransformed cells. Next, their target is identified by mass spectrometry analysis. (B) Determination of the surfaceome of cancer cells expressing an oncogene (e.g., mutant KRAS, KRASMUT) compared to the same cell line not expressing the oncogene (e.g., wild-type KRAS, KRASWT) to discover oncogene-dependent cell surface proteins by mass spectrometry analysis. mAbs are then selected by phage display against these newly discovered proteins.
The first approach consisted of isolating mAbs binding to the surface of cancer cells by phage display (Figure 3A). The mAbs were screened directly on intact cells from seven different carcinomas, including pancreatic cancer cell lines [74]. Then, the antibody clones of interest were selected using fresh tumour tissues by immunohistochemistry. If the clones were binding to malignant cells and not or weakly to normal cells (by checking their staining on the respective type of cells), they would be further characterised by mass spectrometry to identify their target. Using such an approach, a mAb targeting CD147, a transmembrane protein overexpressed in tumours including pancreatic cancer, was shown to induce ADCC and to inhibit the growth of PANC-1 pancreatic cancer cells [74,75]. This antibody was also radiolabelled to monitor pancreatic cancer cells in vivo [76].
In a second approach, the research of novel cell membrane targets was performed first by determining the specific surfaceome of cancer cells. This has been successfully applied to Ewing sarcoma [77] and T cell acute lymphoblastic leukemia (T-ALL) [78] to find cancer cell surface targets and enabled the development of an ADC to selectively kill cancer cells [77].
While the surfaceomes from these studies were determined by RNAseq analysis of cancerous versus non-cancerous cells, another method was developed and used isogenic cell lines [79]. These cell lines allowed the discovery of the cell membrane proteins specifically expressed upon oncogenic KRASG12V expression compared to KRASWT cells by mass spectrometry analysis. After finding the KRAS-regulated surfaceome proteins, recombinant monoclonal antibodies were generated by phage display against seven different membrane-bound proteins, including CUB domain containing protein 1 (CDCP1) (Figure 3B). CDCP1 drives loss of adhesion through integrin signalling [80]. It is overexpressed in various cancers and has been previously involved as a driver of cancer cell growth, metastasis and tumour progression [81]. It is specifically expressed on pancreatic cancer cell lines and not on non-tumorigenic pancreatic ductal cells (HPNE), making this protein an attractive target. Because antibodies are versatile tools, anti-CDCP1 antibody was engineered and used in various set-ups. Notably, it enabled the delivery of an ADC to selectively kill PDAC cells. When engineered in a bispecific T cell engager (BiTE) modality (fused to an anti-CD3 scFv, Figure 2D), the antibody could recruit and activate T cells to PDAC cells while sparing normal cells. Finally, when labelled with a positron-emitting radioisotope (89Zr), the antibody showed efficacy to image the tumour in vivo [79]. This example highlights the power of this approach to discover novel antibodies against membrane proteins that have an expression controlled by an oncoprotein.
3. Targeting Immune Checkpoints
Immune checkpoints are receptors expressed by immune cells that enable dynamic regulation of immune homeostasis and are particularly relevant to T cell functionality. The most studied receptors are programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)/CD80 (also known as B7). When the receptors are in interaction, this leads to T cell exhaustion (i.e., an “inactive” state). This phenomenon is found physiologically to limit autoimmune inflammation or maintain foetal tolerance during pregnancy but is also exploited by cancer cells to maintain immune tolerance.
Therefore, immune checkpoint inhibitors (CPIs) have been developed to inhibit these receptors’ interactions and consequently reactivate the immune system (e.g., T cells) to modulate the immune response against cancer cells [82]. In 2011, ipilimumab, the first antibody blocking an immune checkpoint (CTLA4), was authorized by the FDA. This was rapidly followed by the development of monoclonal antibodies targeting PD-1 (pembrolizumab and nivolumab) and PD-L1 (atezolizumab and durvalumab), which impede PD-1/PD-L1 interaction [83]. T-cell-targeted immunomodulators are now used as single agents or in combination with chemotherapies as first or second lines of treatment for various cancers. This strategy was successful across numerous solid tumours, such as melanoma, non-small cell lung cancer, renal cancer, hepatocellular carcinoma and mismatch repair-deficient metastatic colorectal cancer, producing sustained anti-tumour responses [84]. We will now review in this section the advances and challenges in pancreatic cancer immunotherapy.
Compared to normal pancreatic samples, PD-L1 expression is upregulated in 19% of tumour samples, and it was suggested that CPIs might reactivate exhausted T cells to increase the anti-tumour immune response in PD-L1-upregulated tumours [85]. In addition, CTLA-4 is widely expressed within the TME. In tumour lesions, it is found expressed on infiltrating Tregs, conventional exhausted T cells, or on tumour cells themselves, contributing to an immunosuppressive environment [86,87,88]. Furthermore, blockage of CTLA-4/CD80 interaction is sufficient to induce CD4+ T cell infiltration into pancreatic tumours, demonstrating that the CTLA-4/CD80 axis regulates T cell infiltration in pancreatic cancer [88].
However, the early-phase clinical studies employing either PD-1 [89], PD-L1 mAbs [90] or CTLA-4 mAbs [91] as monotherapy did not show any clinical benefit. These negative clinical studies highlighted the resistance of PDAC to CPIs. This resistance can be explained by the complex immunosuppressive landscape of the PDAC microenvironment. It notably impedes tumour infiltration by effector T cells, making immune quiescent tumours (also called immunologically “cold” tumour) [92], while PDAC cells harbour neoantigens of high quality [93] that are immunoedited with time in long-term survivors [94]. Hence, the focus was next to fight this immunosuppressive TME by using various combination therapies, including dual CPIs therapy, CPIs with chemotherapies, radiotherapies or vaccines [95].
Chemotherapies were initially thought to prevent tumour growth by inhibiting cellular proliferation or inducing cell death. However, recent studies indicate that chemotherapeutic drugs can boost the immunogenicity of tumour cells or cause immunogenic cell death (ICD) in various tumour models [96]. On one hand, gemcitabine can affect the TME through the inhibition of the expansion of immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) [97]. On the other hand, it can also induce the expansion of anti-inflammatory M2 macrophages by a T helper 2 cytokine environment [98], which have been shown to increase the resistance to gemcitabine by upregulating cytidine deaminase level [99]. Hence, ipililumab was tested in a clinical trial (NCT01473940) in combination with gemcitabine and nab-paclitaxel, where its efficacy was comparable to chemotherapy alone [100]. However, combining pembrolizumab, gemcitabine and nab-paclitaxel slightly improved the OS of PDAC patients (NCT02331251) [101]. The main difference between these clinical trials is that the pembrolizumab trial was conducted on chemotherapy naïve patients, while the ipililumab-based trial was not.
Focal adhesion kinase (FAK) is an important regulator of the fibrotic and immunosuppressive TME in PDAC [102]. Its inhibition increased immune surveillance by defeating the stromal fibrosis and immunosuppressive PDAC TME and induced sensitisation to immunotherapy [102]. Therefore, pembrolizumab and a FAK inhibitor, defactinib, are used in clinical trials together (NCT02758587) or combined with gemcitabine (NCT02546531). So far, the results are not available. The same type of clinical trials are ongoing with different chemotherapies and/or antibodies (nivolumab/nab-paclitaxel (NCT02309177), nivolumab/FOLFIRINOX (NCT03970252)), and the outcomes are not published yet.
Interestingly, knowing the benefit of olaparib treatment in platinum-sensitive metastatic PDAC patients with germline BRCA1/2 mutations, a new clinical trial was launched in 2021 to study the combination of pembrolizumab with olaparib in patients with metastatic PDAC with a high tumour mutation burden (NCT05093231).
Radiotherapy (RT) is known to induce ICD [103] and an abscopal effect. This occurs when RT not only shrinks the targeted tumour but also induces tumour regression at non-irradiated, distant sites [104]. Additionally, the combination of local RT and immune-modulation could increase local tumour control and cause distant anti-tumour effects through increased tumour-antigen release and antigen-presenting cell (APC) cross-presentation, improved dendritic-cell (DC) function and enhanced T cell priming [105,106,107]. Actually, preclinical studies showed that RT induces an abscopal tumour-specific immune response in both the irradiated and non-irradiated tumours that is potentiated by PD-1 blockage [108]. A synergic effect of RT and anti-PD-L1 was demonstrated on PDAC mice models [109]. Moreover, in the same study, the authors indicated that RT induces immunosensitisation of tumour cells and that anti-PD-L1 increases recruitment of CD8+ T cells and decreases the establishment of suppressive microenvironment factors [109]. Accordingly, clinical trials evaluate the efficacy of these different combinations of CPI and RT in pancreatic cancer. For example, a phase II trial combining RT, ipilimumab and nivolumab in patients with metastatic microsatellite-stable colorectal or PDAC (25 patients) was performed (NCT03104439). It demonstrated the safety but also the modest efficacy of RT to enhance the effects of dual checkpoint inhibition in MSS metastatic CRC and PDAC [110]. While combining chemotherapy or RT with CPIs only showed limited improvement for the patients so far, studying the potential efficacy of dual checkpoint inhibition (ipilimumab, nivolumab) in combination with gemcitabine and nab-paclitaxel followed by immune-chemoradiation in locally advanced pancreatic cancer (LAPTOP, NCT04247165) might be the solution to obtain a clinical benefit.
Lastly, combination of CPI with vaccines is another therapeutic strategy under investigation in PDAC patients. Indeed, vaccines may have the potential to convert “non-immunogenic” PDAC into an immunogenic tumour through enhanced antigen presentation and priming of antigen-specific T cells. Several vaccines are tested, including GVAX, which is a whole tumour cell vaccine genetically engineered to express granulocyte-macrophage colony-stimulating factor (GM-CSF) [111]. It consists of two irradiated human allogeneic pancreatic tumour cell lines modified to secrete GM-CSF, a cytokine that induces the maturation of dendritic cells. Initially tested as a single agent in cancers, it is now under investigation in combination therapies, including with CPIs. Combination of GVAX vaccine and PD-1 blocking antibody facilitates effector T cell infiltration into pancreatic tumours and, consequently, improved murine survival compared to PD-1 antibody monotherapy or GVAX therapy alone [112]. Accordingly, different clinical trials studied the most efficacious combinations. Unfortunately, the results of the phase II STELLAR trial, where the cancer vaccine GVAX, cyclophosphamide (CY) and CRS-207 (live, attenuated Listeria monocytogenes expressing mesothelin), evaluated with or without nivolumab, were disappointing (NCT02243371). Neither improvement to OS (5.88 vs. 6.11 months) nor significant differences for PFS or time to progression were shown in this clinical study.
Anti-CTLA-4 mAb, ipilimumab, was administered in locally advanced or metastatic pancreatic cancer in combination with GVAX vaccine (NCT00836407). Clinical activity was observed with improved OS (5.7 versus 3.6 months) and 1-year survival (27% versus 7%) in patients that received ipilimumab and GVAX versus ipilimumab alone, indicating the potential efficacy of this combination [113]. However, giving combination GVAX and ipilimumab immediately after front-line chemotherapy (here FOLFIRINOX) in the maintenance setting did not improve OS, but biological effects on immune cells were observed (NCT01896869) [114]. Further study of novel combinations in the maintenance treatment of metastatic PDA is feasible.
Recently, a phase I trial used a sequential treatment: tumour resection, followed by atezolizumab (PD-L1 blockade), followed by a systemic mRNA-based personalised neoantigen-specific immunotherapy vaccine (called autogene cevumeran) and FOLFIRINOX chemotherapy (NCT04161755). The preliminary results recently released are promising: out of 19 patients, 16 received the vaccine and 50% had neoantigen-specific immunity that correlates with improved a PDAC outcome compared with non-responders [115]. These data showed autogene cevumeran is safe, feasibly manufactured in a clinically relevant timeframe and immunogenic in PDAC [115]. The individualised neoantigen tumour vaccines are probably one solution for PDAC patients knowing the high heterogeneity between the patients’ tumours.
The future of immunotherapy will probably rely on the implementation of a personalised vaccine but also on the development of novel agents targeting additional immune checkpoints, co-stimulatory receptors and/or co-inhibitory receptors that control T cell function to improve the efficacy of the current CPIs [116]. In addition, analysis of the tumour-infiltrating lymphocytes (TILs) in PDAC biopsies could help determine the tumour immune status in order to select patients suitable for immunotherapy [117].
4. Targeting Intracellular Proteins
Although PDACs are highly heterogeneous tumours at both the inter- and intra-tumoral genomic level, recurrent genetic and molecular alterations are common traits of this cancer. Those include activating mutations on KRAS (>90% of tumours) [118] and inactivating mutations of TP53, CDKN2A and SMAD4 (50–80%) [2]. Therefore, such proteins could be attractive therapeutic proteins to inhibit or stabilise. However, these are intracellular proteins that remain difficult to target with small molecules, with the exception of the KRASG12C mutation, which is druggable since 2021 with sotorasib [119]. Therefore, alternative strategies may help targeting these proteins. Intracellular antibodies are reagents that could be applied to this objective. Intracellular antibodies are protein binders that are expressed within the cells where they will interact with their target to either track [120,121], inhibit [122,123] or degrade it [124,125].
4.1. KRAS
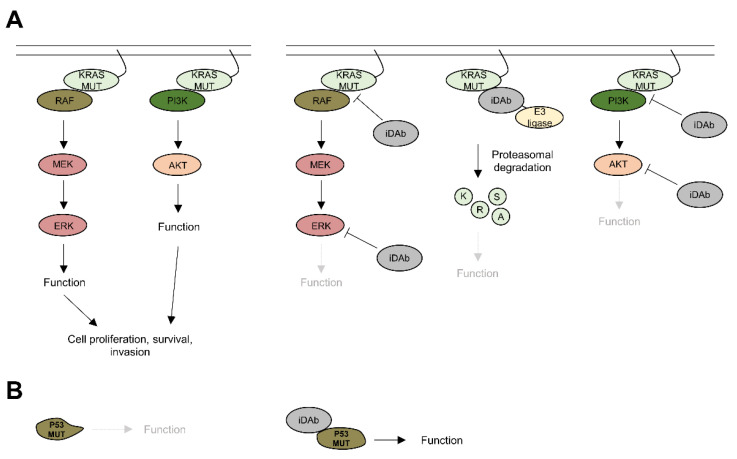
KRAS switches between an inactive GDP-bound state and an active GTP-bound state. When mutated, KRAS persistently activates downstream signalling pathways by interacting with various effector proteins, leading to cell proliferation, survival and/or invasion [126] (Figure 4A). Hence, the first strategy was implemented by the Rabbitts group with the characterisation of an intracellular scFv that binds an RAS-GTP conformation and inhibits RAS transformation in vitro [127]. An intracellular single domain antibody (iDAb) was then developed and showed for the first time the feasibility to impede RAS/effector protein–protein interactions (PPIs) as an effective strategy to inhibit tumour growth and metastasis in vivo [123,128] (Figure 4A). However, the withdrawal of the iDAb led to a restart of the tumour growth [128], suggesting that combination therapies might be needed to effectively induce cancer cell death and avoid the apparition of resistance mechanisms. These data were further supported by the studies of the Kim lab. They developed anti-RAS-GTP intracellular full-length antibodies blocking RAS PPIs, demonstrated that resistance appeared after treating tumours with these molecules and that combination therapies were needed to overcome these resistance mechanisms [129,130].
Figure 4.
Potential of intracellular antibodies in pancreatic cancer. (A) Intracellular antibodies can be used to block protein–protein interactions between KRAS and its effectors, RAF or PI3K, to directly degrade KRAS by targeted protein degradation or to block downstream signalling kinases, such as ERK and AKT, to inhibit cell proliferation, survival or invasion. (B) Intracellular antibodies could be employed to restore the function of tumour suppressors, such as P53, by modifying their conformation.
One issue with PPI inhibitors is their mode of action that is occupancy-driven, where one inhibitor inhibits one target. Targeting protein degradation offers the advantage of working by an event-driven mode of action, which means that one degrader can deplete several targets (i.e., catalytic mechanism). Hence, the antibody-based degrader technology is a promising therapeutic strategy. This was highlighted by the functionalisation of both the anti-RAS iDAb with the UBOX domain from the CHIP E3 ubiquitin ligase and the anti-KRAS antibody mimetic binder [122] with the von Hippel–Lindau (VHL) as antibody-based degraders [125]. These RAS degraders have shown efficacy in all cell lines tested, including pancreatic cancer cells. They efficiently depleted RAS/KRAS proteins within a few hours, consequently inhibited RAS downstream signalling pathways and induced cancer cell death by apoptosis. In vivo, these degraders led to the rapid regression of mutant (K)RAS tumours, suggesting that the targeted degradation of (K)RAS is an attractive therapeutic strategy [125] (Figure 4A).
The persistent activation of mutated KRAS in PDAC leads to the activation of downstream signalling pathways, such as mitogen-activated protein kinase (MAPK) and the PI3K pathways. Therefore, targeting downstream mediators of RAS signalling combined (or not) with KRAS inhibition, for instance, could be another possibility. Several intracellular antibodies or antibody mimetics have been developed towards these RAS downstream mediators.
4.2. AKT
AKT is a kinase family that includes three isoforms (AKT1, 2 and 3). While AKT1 and AKT2 are ubiquitously expressed, AKT3 is predominantly found in the heart, brain and kidney [131]. AKT activation is one of the most common molecular alterations in human cancers and regulates cell proliferation and survival but also response to nutrient availability and protein synthesis, which are hallmarks of cancers [132]. Hence, several groups developed anti-AKT intracellular antibodies (Figure 4A). Pan-AKT inhibition with a scFv was previously reported, and its expression within cells led to apoptosis in vitro and in vivo [133]. Because pan-AKT inhibition can induce unwanted toxicities, a more specific inhibition of AKT isoforms was achieved, with nanobodies specifically targeting either AKT1 [134] or AKT2 [135], and it showed inhibitory effects in vitro, but additional works would be required to check their efficacy in vivo.
4.3. ERK
ERK1/2 is a kinase belonging to the MAPK pathway that is involved in the signal transduction into the nucleus to activate numerous transcription factors, such as FOS, JUN or MYC, that ultimately control cell proliferation [136]. Antibody mimetic binders targeting either ERK1/2 or the phosphorylated ERK1/2 were developed (Figure 4A), but their potency to inhibit cell proliferation in vitro and tumour growth in vivo needs to be assessed [137].
4.4. Alternative Strategies
Mutated tumour suppressors, such as TP53, can have their conformation modified and, consequently, be inactivated or have a decreased expression [138]. Therefore, restoring the activity of a mutant tumour suppressor with intracellular antibodies is an attractive therapeutic possibility that would be worth exploring. Actually, this has been achieved with scFvs targeting mutant P53 and restoring its activity in vitro [139,140,141], but this strategy was not tested in vivo. Nevertheless, small peptides targeting mutant P53 restored the WT conformation to mutant P53 with in vivo activity [142], showing the feasibility of such an approach in a preclinical setting (Figure 4B).
5. Conclusions and Future Directions
Pancreatic cancer is an aggressive cancer with limited treatment options that has only modest clinical responses. Therapeutic monoclonal antibodies have been successful in many cancers, but, as we discussed in this review, they also have limited efficacy in PDAC as monotherapy most likely due to the heterogeneity found in PDAC tumours. Therefore, (i) there is a need to discover a novel target for therapy, and the development of strategies, such as the surfaceome, could be advantageous. (ii) The combination of several therapeutic strategies (including mAbs) is most likely the future of PDAC treatment, with, notably, personalised medicine, such as vaccines, to overcome the heterogeneity issue [143]. Several clinical trials are ongoing with a combination of different mAbs, such as the phase I/II trial that is currently recruiting patients and employs the anti-MSLN ADC (anetumab ravtansine) with an anti-PD1 (nivolumab) and/or anti-CTLA-4 (ipilimumab) and/or gemcitabine (NCT03816358). (iii) As discussed in this review, while non-modified mAbs showed limited efficacy, their functionalisation into ADC or radiolabelled mAbs could enhance their efficacy. However, not all mAbs can be modified, particularly because their target needs to be internalised for ADC or targeted radionuclide therapy to work and not all cell surface proteins internalise. One cause of the little effect of the mAbs is the dense TME found in PDAC, which limits the tumour accessibility. Consequently, therapeutic strategies should include molecules that interfere with this TME, such as targeting a subset of cancer-associated fibroblasts (CAF) [144] or their secreted products (e.g., TGF-β) [145].
Nevertheless, using mAbs for the diagnostic or visualisation of the tumours is promising, as revealed by the development of SGM-101-modified mAb for image-guided surgery. Again, the discovery of novel biomarkers will help to increase the accuracy of tumours imaging. For instance, it would be of interest to discover specific cell surface proteins on pancreatic cancer tissue at an early stage (e.g., PanIN lesions) to improve the diagnostic/tumour imaging, but this requires the availability of patient tissue at an early stage.
One major issue with PDAC tumours is their “cold” immunogenic property, which impedes the direct use of CPIs that demonstrated great results for other cancers. While personalised vaccines might overcome this problem, other solutions are studied to make the tumours immunogenic, such as oncolytic viruses [146].
Finally, the implementation of intracellular antibodies is far from reaching the clinic yet, but this application is promising as it could directly interfere with the main oncoproteins or tumour suppressors that are deregulated in PDAC. The major hurdle to pass is the delivery inside the cells of such reagents. This is under development, with different strategies being investigated, such as viral or non-viral delivery strategies [9]. The latter is notably promising because it has been successfully employed to deliver mRNA, encoding the trimerized receptor-binding domain of the spike glycoprotein of SARS-CoV-2 with lipid nanoparticles for COVID-19 mRNA vaccine [147]. Another way of using intracellular antibodies has been developed by the Rabbitts group with the antibody-derived compound (Abd) strategy. Abd uses intracellular antibodies as a guide to select small molecules that would display the same inhibitory mechanism as the intracellular antibody. This strategy has been successfully applied to LMO2 and RAS oncoproteins [148,149,150,151,152,153], with notable antiproliferative and cytotoxic effects for the anti-RAS compounds in RAS-mutated cancer cell lines.
Author Contributions
M.S., P.C. and N.B. wrote the paper; M.S. and N.B. made all the figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M.S. is supported by a fellowship from La Ligue Nationale Contre le Cancer. N.B. is supported by a fellowship from the Fondation de France (N°00097692).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., et al. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Christine Cripps M., Portenoy R.K., Storniolo A.M., Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardière C., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffitt R.A., Marayati R., Flate E.L., Volmar K.E., Loeza S., Hoadley K.A., Rashid N.U., Williams L.A., Eaton S.C., Chung A.H., et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puleo F., Nicolle R., Blum Y., Cros J., Marisa L., Demetter P., Quertinmont E., Svrcek M., Elarouci N., Iovanna J., et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018;155:1999–2013. doi: 10.1053/j.gastro.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.O., Hochhauser D., Arnold D., Oh D.Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Ötjengerdes R.M., Roewe J., Mejias-Estevez R., Marschall A.L.J. Applying Antibodies Inside Cells: Principles and Recent Advances in Neurobiology, Virology and Oncology. BioDrugs. 2020;34:435–462. doi: 10.1007/s40259-020-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner L.M., Surana R., Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drago J.Z., Modi S., Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021;18:327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parakh S., Lee S.T., Gan H.K., Scott A.M. Radiolabeled Antibodies for Cancer Imaging and Therapy. Cancers. 2022;14:1454. doi: 10.3390/cancers14061454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobson L., Reményi I., Tusnády G.E. The human transmembrane proteome. Biol. Direct. 2015;10:31. doi: 10.1186/s13062-015-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieduwilt M.J., Moasser M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008;65:1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., Au H.J., Murawa P., Walde D., Wolff R.A., et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 16.Overholser J.P., Prewett M.C., Hooper A.T., Waksal H.W., Hicklin D.J. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89:74–82. doi: 10.1002/1097-0142(20000701)89:1<74::AID-CNCR11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Graham J., Muhsin M., Kirkpatrick P. Cetuximab . Nat. Rev. Drug. Discov. 2004;3:549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 18.Bruns C.J., Harbison M.T., Davis D.W., A Portera C., Tsan R., McConkey D.J., Evans D.B., Abbruzzese J.L., Hicklin D.J., Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin. Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 19.Philip P.A., Benedetti J., Corless C.L., Wong R., O’Reilly E.M., Flynn P.J., Rowland K.M., Atkins J.N., Mirtsching B.C., Rivkin S.E., et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J. Clin. Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu G., van den Berg N.S., Martin B.A., Nishio N., Hart Z.P., van Keulen S., Fakurnejad S., Chirita S.U., Raymundo R.C., Yi G., et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020;5:753–764. doi: 10.1016/S2468-1253(20)30088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z., Wang M., Yao X., Luo W., Qu Y., Yu D., Li X., Fang J., Huang C. Development of a Novel EGFR-Targeting Antibody-Drug Conjugate for Pancreatic Cancer Therapy. Target. Oncol. 2019;14:93–105. doi: 10.1007/s11523-018-0616-8. [DOI] [PubMed] [Google Scholar]
- 22.McDaid W.J., Greene M.K., Johnston M.C., Pollheimer E., Smyth P., McLaughlin K., Van Schaeybroeck S., Straubinger R.M., Longley D.B., Scott C.J. Repurposing of Cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumours. Nanoscale. 2019;11:20261–20273. doi: 10.1039/C9NR07257H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reusch U., Sundaram M., Davol P.A., Olson S.D., Davis J.B., Demel K., Nissim J., Rathore R., Liu P.Y., Lum L.G. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 2006;12:183–190. doi: 10.1158/1078-0432.CCR-05-1855. [DOI] [PubMed] [Google Scholar]
- 24.Lum L.G., Thakur A., Choi M., Deol A., Kondadasula V., Schalk D., Fields K., Dufrense M., Philip P., Dyson G., et al. Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. OncoImmunology. 2020;9:1773201. doi: 10.1080/2162402X.2020.1773201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blasco M.T., Navas C., Martín-Serrano G., Graña-Castro O., Lechuga C.G., Martín-Díaz L., Djurec M., Li J., Morales-Cacho L., Esteban-Burgos L., et al. Complete Regression of Advanced Pancreatic Ductal Adenocarcinomas upon Combined Inhibition of EGFR and C-RAF. Cancer Cell. 2019;35:573–587. doi: 10.1016/j.ccell.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bera T.K., Pastan I. Mesothelin Is Not Required for Normal Mouse Development or Reproduction. Mol. Cell. Biol. 2000;20:2902–2906. doi: 10.1128/MCB.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argani P., Iacobuzio-Donahue C., Ryu B., Rosty C., Goggins M., E Wilentz R., Murugesan S.R., Leach S.D., Jaffee E., Yeo C.J., et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin. Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 28.Chowdhury P.S., Vasmatzis G., Beers R., Lee B., Pastan I. Improved stability and yield of a Fv-toxin fusion protein by computer design and protein engineering of the Fv. J. Mol. Biol. 1998;281:917–928. doi: 10.1006/jmbi.1998.1980. [DOI] [PubMed] [Google Scholar]
- 29.Hassan R., Williams-Gould J., Steinberg S.M., Liewehr D.J., Yokokawa J., Tsang K.Y., Surawski R.J., Scott T., Camphausen K., Xin X., et al. Tumor-Directed Radiation and the Immunotoxin SS1P in the Treatment of Mesothelin-Expressing Tumor Xenografts. Clin. Cancer Res. 2006;12:4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Xiang L., Hassan R., Paik C.H., Carrasquillo J.A., Jang B.-S., Le N., Ho M., Pastan I. Synergistic Antitumor Activity of Taxol and Immunotoxin SS1P in Tumor-Bearing Mice. Clin. Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 31.Hassan R., Broaddus V.C., Wilson S., Liewehr D.J., Zhang J. Anti–Mesothelin Immunotoxin SS1P in Combination with Gemcitabine Results in Increased Activity against Mesothelin-Expressing Tumor Xenografts. Clin. Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 32.Hassan R., Bullock S., Premkumar A., Kreitman R.J., Kindler H., Willingham M.C., Pastan I. Phase I Study of SS1P, a Recombinant Anti-Mesothelin Immunotoxin Given as a Bolus I.V. Infusion to Patients with Mesothelin-Expressing Mesothelioma, Ovarian, and Pancreatic Cancers. Clin. Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 33.Mossoba M.E., Onda M., Taylor J., Massey P.R., Treadwell S., Sharon E., Hassan R., Pastan I., Fowler D.H. Pentostatin Plus Cyclophosphamide Safely and Effectively Prevents Immunotoxin Immunogenicity in Murine Hosts. Clin. Cancer Res. 2011;17:3697–3705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollevoet K., Mason-Osann E., Liu X.F., Imhof-Jung S., Niederfellner G., Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol. Cancer Ther. 2014;13:2040–2049. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolyvas E., Rudloff M., Poruchynsky M., Landsman R., Hollevoet K., Venzon D., Alewine C. Mesothelin-targeted immunotoxin RG7787 has synergistic anti-tumor activity when combined with taxanes. Oncotarget. 2016;8:9189–9199. doi: 10.18632/oncotarget.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alewine C., Ahmad M., Peer C.J., Hu Z.I., Lee M.-J., Yuno A., Kindrick J.D., Thomas A., Steinberg S.M., Trepel J.B., et al. Phase I/II Study of the Mesothelin-targeted Immunotoxin LMB-100 with Nab-Paclitaxel for Patients with Advanced Pancreatic Adenocarcinoma. Clin. Cancer Res. 2020;26:828–836. doi: 10.1158/1078-0432.CCR-19-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan R., Blumenschein G.R., Jr., Moore K.N., Santin A.D., Kindler H.L., Nemunaitis J.J., Seward S.M., Thomas A., Kim S.K., Rajagopalan P., et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020;38:1824–1835. doi: 10.1200/JCO.19.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan R., Ebel W., Routhier E.L., Patel R., Kline J.B., Zhang J., Chao Q., Jacob S., Turchin H., Gibbs L., et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 39.Gubbels J.A., Belisle J., Onda M., Rancourt C., Migneault M., Ho M., Bera T.K., Connor J., Sathyanarayana B.K., Lee B., et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizukami T., Kamachi H., Fujii Y., Matsuzawa F., Einama T., Kawamata F., Kobayashi N., Hatanaka Y., Taketomi A. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget. 2018;9:33844–33852. doi: 10.18632/oncotarget.26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan R., Cohen S.J., Phillips M., Pastan I., Sharon E., Kelly R.J., Schweizer C., Weil S., Laheru D. Phase I Clinical Trial of the Chimeric Anti-Mesothelin Monoclonal Antibody MORAb-009 in Patients with Mesothelin-Expressing Cancers. Clin. Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindenberg L., Thomas A., Adler S., Mena E., Kurdziel K., Maltzman J., Wallin B., Hoffman K., Pastan I., Paik C.H., et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using Single Photon Emission Computed Tomography-Computed Tomography (SPECT-CT) imaging. Oncotarget. 2015;6:4496–4504. doi: 10.18632/oncotarget.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akce M., Zaidi M.Y., Waller E.K., El-Rayes B.F., Lesinski G.B. The Potential of CAR T Cell Therapy in Pancreatic Cancer. Front. Immunol. 2018;9:2166. doi: 10.3389/fimmu.2018.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beatty G.L., O’Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M., et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko A.H., Jordan A.C., Tooker E., Lacey S.F., Chang R.B., Li Y., Venook A.P., Tempero M., Damon L., Fong L., et al. Dual Targeting of Mesothelin and CD19 with Chimeric Antigen Receptor-Modified T Cells in Patients with Metastatic Pancreatic. Cancer Mol. Ther. 2020;28:2367–2378. doi: 10.1016/j.ymthe.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jonckheere N., Skrypek N., Van Seuningen I. Mucins and Pancreatic Cancer. Cancers. 2010;2:1794–1812. doi: 10.3390/cancers2041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh H., Pillai K., Morris D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am. J. Cancer Res. 2017;7:1372–1383. [PMC free article] [PubMed] [Google Scholar]
- 48.Jonckheere N., Skrypek N., Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim. et Biophys. Acta. 2014;1846:142–151. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Wang S., You L., Dai M., Zhao Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell. Mol. Med. 2020;24:10279–10289. doi: 10.1111/jcmm.15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danielczyk A., Stahn R., Faulstich D., Löffler A., Märten A., Karsten U., Goletz S. PankoMab: A potent new generation anti-tumour MUC1 antibody. Cancer Immunol. Immunother. 2006;55:1337–1347. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiedler W., DeDosso S., Cresta S., Weidmann J., Tessari A., Salzberg M., Dietrich B., Baumeister H., Goletz S., Gianni L., et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur. J. Cancer. 2016;63:55–63. doi: 10.1016/j.ejca.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Daiichi Sankyo Enters Worldwide Licensing Agreement with Glycotope for Gatipotuzumab Antibody Drug Conjugate. [(accessed on 1 June 2022)]. Available online: https://www.daiichisankyo.com/media/press_release/detail/index_3243.html.
- 53.Gold D.V., Lew K., Maliniak R., Hernandez M., Cardillo T. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int. J. Cancer. 1994;57:204–210. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- 54.Gold D.V., Cardillo T., Goldenberg D.M., Sharkey R.M. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Crit. Rev. Oncol. Hematol. 2001;39:147–154. doi: 10.1016/S1040-8428(01)00114-7. [DOI] [PubMed] [Google Scholar]
- 55.Cardillo T.M., Ying Z., Gold D.V. Therapeutic advantage of (90)yttrium- versus (131)iodine-labeled PAM4 antibody in experimental pancreatic cancer. Clin. Cancer Res. 2001;7:3186–3192. [PubMed] [Google Scholar]
- 56.Gold D.V., Karanjawala Z., Modrak D.E., Goldenberg D.M., Hruban R.H. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin. Cancer Res. 2007;13:7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 57.Sørensen A.L., Reis C.A., Tarp M.A., Mandel U., Ramachandran K., Sankaranarayanan V., Schwientek T., Graham R., Taylor-Papadimitriou J., Hollingsworth M.A., et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 58.Posey A.D., Jr., Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B., Stone J.D., Madsen T.D., Schreiber K., Haines K.M., et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gautam S.K., Kumar S., Cannon A., Hall B., Bhatia R., Nasser M.W., Mahapatra S., Batra S.K., Jain M. MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin. Ther. Targets. 2017;21:657–669. doi: 10.1080/14728222.2017.1323880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bafna S., Kaur S., Momi N., Batra S.K. Pancreatic cancer cells resistance to gemcitabine: The role of MUC4 mucin. Br. J. Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manne A., Esnakula A., Abushahin L., Tsung A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers. 2021;13:3059. doi: 10.3390/cancers13123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawada T., Nishihara T., Yamamoto A., Teraoka H., Yamashita Y., Okamura T., Ochi H., Ho J.J.L., Kim Y.-S., Hirakawa K. Preoperative Clinical Radioimmunodetection of Pancreatic Cancer by111In-labeled Chimeric Monoclonal Antibody Nd2. Jpn. J. Cancer Res. 1999;90:1179–1186. doi: 10.1111/j.1349-7006.1999.tb00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakata N., Kobashi N., Okumura Y., Sato M., Matono M., Otsuki K., Tanaka A., Hayashi A. Radiation dosimetry and efficacy of an 89Zr/225Ac-labeled humanized anti-MUC5AC antibody. Nucl. Med. Biol. 2022;108:33–43. doi: 10.1016/j.nucmedbio.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Turner M.A., Hollandsworth H.M., Nishino H., Amirfakhri S., Lwin T.M., Lowy A.M., Kaur S., Natarajan G., Mallya K., Hoffman R.M., et al. Fluorescent Anti-MUC5AC Brightly Targets Pancreatic Cancer in a Patient-derived Orthotopic Xenograft. Vivo. 2021;36:57–62. doi: 10.21873/invivo.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni X.G., Bai X.F., Mao Y.L., Shao Y.F., Wu J.X., Shan Y., Wang C.F., Wang J., Tian Y.T., Liu Q., et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur. J. Surg. Oncol. 2005;31:164–169. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Rizeq B., Zakaria Z., Ouhtit A. Towards understanding the mechanisms of actions of carcinoembryonic antigen-related cell adhesion molecule 6 in cancer progression. Cancer Sci. 2018;109:33–42. doi: 10.1111/cas.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Dam M.A., Vuijk F.A., Stibbe J.A., Houvast R.D., Luelmo S.A., Crobach S., Shahbazi Feshtali S., de Geus-Oei L.F., Bonsing B.A., Sier C.F., et al. Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy. Cancers. 2021;13:6088. doi: 10.3390/cancers13236088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato H., Kishiwada M., Hayasaki A., Chipaila J., Maeda K., Noguchi D., Gyoten K., Fujii T., Iizawa Y., Tanemura A., et al. Role of Serum Carcinoma Embryonic Antigen (CEA) Level in Localized Pancreatic Adenocarcinoma: CEA Level Before Operation is a Significant Prognostic Indicator in Patients with Locally Advanced Pancreatic Cancer Treated With Neoadjuvant Therapy Followed by Surgical Resection: A Retrospective Analysis. Ann. Surg. 2022;275:e698–e707. doi: 10.1097/SLA.0000000000004148. [DOI] [PubMed] [Google Scholar]
- 69.Knutson S., Raja E., Bomgarden R., Nlend M., Chen A., Kalyanasundaram R., Desai S. Development and Evaluation of a Fluorescent Antibody-Drug Conjugate for Molecular Imaging and Targeted Therapy of Pancreatic Cancer. PLoS ONE. 2016;11:e0157762. doi: 10.1371/journal.pone.0157762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lwin T.M., Murakami T., Miyake K., Yazaki P.J., Shivley J.E., Hoffman R.M., Bouvet M. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann. Surg. Oncol. 2018;25:1079–1085. doi: 10.1245/s10434-018-6344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutowski M., Framery B., Boonstra M.C., Garambois V., Quenet F., Dumas K., Scherninski F., Cailler F., Vahrmeijer A.L., Pèlegrin A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg. Oncol. 2017;26:153–162. doi: 10.1016/j.suronc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Hoogstins C.E.S., Boogerd L.S.F., Mulder B.G.S., Mieog J.S.D., Swijnenburg R.J., Van De Velde C.J.H., Farina Sarasqueta A., Bonsing B.A., Framery B., Pèlegrin A., et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018;25:3350–3357. doi: 10.1245/s10434-018-6655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lwin T.M., Turner M.A., Nishino H., Amirfakhri S., Hernot S., Hoffman R.M., Bouvet M. Fluorescent Anti-CEA Nanobody for Rapid Tumor-Targeting and Imaging in Mouse Models of Pancreatic Cancer. Biomolecules. 2022;12:711. doi: 10.3390/biom12050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurosawa G., Akahori Y., Morita M., Sumitomo M., Sato N., Muramatsu C., Eguchi K., Matsuda K., Takasaki A., Tanaka M., et al. Comprehensive screening for antigens overexpressed on carcinomas via isolation of human mAbs that may be therapeutic. Proc. Natl. Acad. Sci. USA. 2008;105:7287–7292. doi: 10.1073/pnas.0712202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurosawa G., Sumitomo M., Ukai Y., Subere J., Muramatsu C., Eguchi K., Tanaka-Hashiba M., Sugiura M., Ando M., Sato N., et al. Selection and analysis of anti-cancer antibodies for cancer therapy obtained from antibody phage library. Cancer Sci. 2010;102:175–181. doi: 10.1111/j.1349-7006.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- 76.Sugyo A., Tsuji A.B., Sudo H., Nagatsu K., Koizumi M., Ukai Y., Kurosawa G., Zhang M.-R., Kurosawa Y., Saga T. Evaluation of 89Zr-Labeled Human Anti-CD147 Monoclonal Antibody as a Positron Emission Tomography Probe in a Mouse Model of Pancreatic Cancer. PLoS ONE. 2013;8:e61230. doi: 10.1371/journal.pone.0061230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Town J., Pais H., Harrison S., Stead L.F., Bataille C., Bunjobpol W., Zhang J., Rabbitts T.H. Exploring the surfaceome of Ewing sarcoma identifies a new and unique therapeutic target. Proc. Natl. Acad. Sci. USA. 2016;113:3603–3608. doi: 10.1073/pnas.1521251113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pais H., Ruggero K., Zhang J., Al-Assar O., Bery N., Bhuller R., Weston V., Kearns P., Mecucci C., Miller A., et al. Surfaceome interrogation using an RNA-seq approach highlights leukemia initiating cell biomarkers in an LMO2 T cell transgenic model. Sci. Rep. 2019;9:5760. doi: 10.1038/s41598-019-42214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinko A.J., Truillet C., Julien O., Diaz J.E., Horlbeck M.A., Whiteley G., Blonder J., Weissman J.S., Bandyopadhyay S., Evans M.J., et al. Targeting RAS-driven human cancer cells with antibodies to upregulated and essential cell-surface proteins. eLife. 2018;7:e31098. doi: 10.7554/eLife.31098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhatt A.S., Erdjument-Bromage H., Tempst P., Craik C.S., Moasser M.M. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Casar B., Rimann I., Kato H., Shattil S.J., Quigley J.P., Deryugina E.I. In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated beta1 integrin and induction of FAK/PI3K/Akt motility signaling. Oncogene. 2014;33:255–268. doi: 10.1038/onc.2012.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esfahani K., Roudaia L., Buhlaiga N., Del Rincon S.V., Papneja N., Miller W.H., Jr. A Review of Cancer Immunotherapy: From the Past to the Present, to the Future. Curr. Oncol. 2020;27:S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birnbaum D.J., Finetti P., Lopresti A., Gilabert M., Poizat F., Turrini O., Raoul J.L., Delpero J.R., Moutardier V., Birnbaum D., et al. Prognostic value of PDL1 expression in pancreatic cancer. Oncotarget. 2016;7:71198–71210. doi: 10.18632/oncotarget.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contardi E., Palmisano G.L., Tazzari P.L., Martelli A.M., Falà F., Fabbi M., Kato T., Lucarelli E., Donati D., Polito L., et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 87.Sobhani N., Tardiel-Cyril D.R., Davtyan A., Generali D., Roudi R., Li Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers. 2021;13:1440. doi: 10.3390/cancers13061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bengsch F., Knoblock D.M., Liu A., McAllister F., Beatty G.L. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother. 2017;66:1609–1617. doi: 10.1007/s00262-017-2053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patnaik A., Kang S.P., Rasco D., Papadopoulos K.P., Elassaiss-Schaap J., Beeram M., Drengler R., Chen C., Smith L., Espino G., et al. Phase I Study of Pembrolizumab (MK-3475; Anti–PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 90.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmiechen Z.C., Stromnes I.M. Mechanisms Governing Immunotherapy Resistance in Pancreatic Ductal Adenocarcinoma. Front Immunol. 2020;11:613815. doi: 10.3389/fimmu.2020.613815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balachandran V.P., Łuksza M., Zhao J.N., Makarov V., Moral J.A., Remark R., Herbst B., Askan G., Bhanot U., Senbabaoglu Y., et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Łuksza M., Sethna Z.M., Rojas L.A., Lihm J., Bravi B., Elhanati Y., Soares K., Amisaki M., Dobrin A., Hoyos D., et al. Neoantigen quality predicts immunoediting in survivors of pancreatic cancer. Nature. 2022;606:389–395. doi: 10.1038/s41586-022-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong J., Hendifar A., Tuli R., Chuang J., Cho M., Chung V., Li D., Salgia R. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: Overcoming resistance to single-agent checkpoint blockade. Clin. Transl. Med. 2018;7:32. doi: 10.1186/s40169-018-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown J.S., Sundar R., Lopez J. Combining DNA damaging therapeutics with immunotherapy: More haste, less speed. Br. J. Cancer. 2017;118:312–324. doi: 10.1038/bjc.2017.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gabitass R.F., Annels N.E., Stocken D.D., Pandha H.A., Middleton G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Q., Li Y., Niu Z., Zong Y., Wang M., Yao L., Lu Z., Liao Q., Zhao Y. Atorvastatin (Lipitor) attenuates the effects of aspirin on pancreatic cancerogenesis and the chemotherapeutic efficacy of gemcitabine on pancreatic cancer by promoting M2 polarized tumor associated macrophages. J. Exp. Clin. Cancer Res. 2016;35:33. doi: 10.1186/s13046-016-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amit M., Gil Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. OncoImmunology. 2013;2:e27231. doi: 10.4161/onci.27231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamath S.D., Kalyan A., Kircher S., Nimeiri H., Fought A.J., Benson A., Mulcahy M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. 2019;25:e808–e815. doi: 10.1634/theoncologist.2019-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiss G.J., Blaydorn L., Beck J., Bornemann-Kolatzki K., Urnovitz H., Schütz E., Khemka V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs. 2018;36:96–102. doi: 10.1007/s10637-017-0525-1. [DOI] [PubMed] [Google Scholar]
- 102.Jiang H., Hegde S., Knolhoff B.L., Zhu Y., Herndon J.M., Meyer M.A., Nywening T.M., Hawkins W.G., Shapiro I.M., Weaver D.T., et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Procureur A., Simonaggio A., Bibault J.-E., Oudard S., Vano Y.-A. Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers. 2021;13:678. doi: 10.3390/cancers13040678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daguenet E., Louati S., Wozny A.-S., Vial N., Gras M., Guy J.-B., Vallard A., Rodriguez-Lafrasse C., Magné N. Radiation-induced bystander and abscopal effects: Important lessons from preclinical models. Br. J. Cancer. 2020;123:339–348. doi: 10.1038/s41416-020-0942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharabi A.B., Nirschl C.J., Kochel C.M., Nirschl T.R., Francica B.J., Velarde E., Deweese T.L., Drake C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gupta A., Probst H.C., Vuong V., Landshammer A., Muth S., Yagita H., Schwendener R., Pruschy M., Knuth A., van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 107.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R., Fu Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park S.S., Dong H., Liu X., Harrington S.M., Krco C.J., Grams M.P., Mansfield A.S., Furutani K.M., Olivier K.R., Kwon E.D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Azad A., Yin Lim S., D’Costa Z., Jones K., Diana A., Sansom O.J., Kruger P., Liu S., McKenna W.G., Dushek O., et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 2017;9:167–180. doi: 10.15252/emmm.201606674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parikh A.R., Szabolcs A., Allen J.N., Clark J.W., Wo J.Y., Raabe M., Thel H., Hoyos D., Mehta A., Arshad S., et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat. Cancer. 2021;2:1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R.C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soares K.C., Rucki A.A., Wu A.A., Olino K., Xiao Q., Chai Y., Wamwea A., Bigelow E., Lutz E., Liu L., et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S., Zheng L., Diaz L.A., Jr., Donehower R.C., Jaffee E.M., et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu A.A., Bever K.M., Ho W.J., Fertig E.J., Niu N., Zheng L., Parkinson R.M., Durham J.N., Onners B.L., Ferguson A.K., et al. A Phase II Study of Allogeneic GM-CSF–Transfected Pancreatic Tumor Vaccine (GVAX) with Ipilimumab as Maintenance Treatment for Metastatic Pancreatic Cancer. Clin. Cancer Res. 2020;26:5129–5139. doi: 10.1158/1078-0432.CCR-20-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Balachandran V.P., Rojas L.A., Sethna Z., Soares K., Derhovanessian E., Mueller F., Yadav M., Basturk O., Gonen M., Wei A.C.-C., et al. Phase I trial of adjuvant autogene cevumeran, an individualized mRNA neoantigen vaccine, for pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2022;40:2516. doi: 10.1200/JCO.2022.40.16_suppl.2516. [DOI] [Google Scholar]
- 116.Kraehenbuehl L., Weng C.-H., Eghbali S., Wolchok J.D., Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2021;19:37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 117.Ino Y., Oguro S., Yamazaki-Itoh R., Hori S., Shimada K., Hiraoka N. Reliable evaluation of tumor-infiltrating lymphocytes in pancreatic cancer tissue biopsies. Oncotarget. 2019;10:1149–1159. doi: 10.18632/oncotarget.26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buscail L., Bournet B., Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 119.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 120.Jullien D., Vignard J., Fedor Y., Bery N., Olichon A., Crozatier M., Erard M., Cassard H., Ducommun B., Salles B., et al. Chromatibody, a novel non-invasive molecular tool to explore and manipulate chromatin in living cells. J. Cell Sci. 2016;129:2673–2683. doi: 10.1242/jcs.183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panza P., Maier J., Schmees C., Rothbauer U., Söllner C. Live imaging of endogenous protein dynamics in zebrafish using chromobodies. Development. 2015;142:1879–1884. doi: 10.1242/dev.118943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bery N., Legg S., Debreczeni J., Breed J., Embrey K., Stubbs C., Kolasinska-Zwierz P., Barrett N., Marwood R., Watson J., et al. KRAS-specific inhibition using a DARPin binding to a site in the allosteric lobe. Nat. Commun. 2019;10:2607. doi: 10.1038/s41467-019-10419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka T., Williams R.L., Rabbitts T.H. Tumour prevention by a single antibody domain targeting the interaction of signal transduction proteins with RAS. EMBO J. 2007;26:3250–3259. doi: 10.1038/sj.emboj.7601744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bery N., Keller L., Soulié M., Gence R., Iscache A.-L., Cherier J., Cabantous S., Sordet O., Lajoie-Mazenc I., Pedelacq J.-D., et al. A Targeted Protein Degradation Cell-Based Screening for Nanobodies Selective toward the Cellular RHOB GTP-Bound Conformation. Cell Chem. Biol. 2019;26:1544–1558. doi: 10.1016/j.chembiol.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 125.Bery N., Miller A., Rabbitts T. A potent KRAS macromolecule degrader specifically targeting tumours with mutant KRAS. Nat. Commun. 2020;11:3233. doi: 10.1038/s41467-020-17022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug. Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka T., Rabbitts T.H. Intrabodies based on intracellular capture frameworks that bind the RAS protein with high affinity and impair oncogenic transformation. EMBO J. 2003;22:1025–1035. doi: 10.1093/emboj/cdg106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tanaka T., Rabbitts T.H. Interfering with RAS–effector protein interactions prevent RAS-dependent tumour initiation and causes stop–start control of cancer growth. Oncogene. 2010;29:6064–6070. doi: 10.1038/onc.2010.346. [DOI] [PubMed] [Google Scholar]
- 129.Shin S.M., Choi D.K., Jung K., Bae J., Kim J.S., Park S.W., Song K.H., Kim Y.S. Antibody targeting intracellular oncogenic Ras mutants exerts anti-tumour effects after systemic administration. Nat. Commun. 2017;8:15090. doi: 10.1038/ncomms15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shin S.-M., Kim J.-S., Park S.-W., Jun S.-Y., Kweon H.-J., Choi D.-K., Lee D., Cho Y.B., Kim Y.-S. Direct targeting of oncogenic RAS mutants with a tumor-specific cytosol-penetrating antibody inhibits RAS mutant–driven tumor growth. Sci. Adv. 2020;6:eaay2174. doi: 10.1126/sciadv.aay2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bellacosa A., Kumar C.C., Di Cristofano A., Testa J.R. Activation of AKT Kinases in Cancer: Implications for Therapeutic Targeting. Adv. Cancer Res. 2005;94:29–86. doi: 10.1016/s0065-230x(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 132.Song M., Bode A.M., Dong Z., Lee M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 133.Shin I., Edl J., Biswas S., Lin P.C., Mernaugh R., Arteaga C.L. Proapoptotic Activity of Cell-Permeable Anti-Akt Single-Chain Antibodies. Cancer Res. 2005;65:2815–2824. doi: 10.1158/0008-5472.CAN-04-2898. [DOI] [PubMed] [Google Scholar]
- 134.Merckaert T., Zwaenepoel O., Gevaert K., Gettemans J. Development and characterization of protein kinase B/AKT isoform-specific nanobodies. PLoS ONE. 2020;15:e0240554. doi: 10.1371/journal.pone.0240554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Merckaert T., Zwaenepoel O., Gevaert K., Gettemans J. An AKT2-specific nanobody that targets the hydrophobic motif induces cell cycle arrest, autophagy and loss of focal adhesions in MDA-MB-231 cells. Biomed. Pharmacother. 2020;133:111055. doi: 10.1016/j.biopha.2020.111055. [DOI] [PubMed] [Google Scholar]
- 136.Klomp J.E., Klomp J.A., Der C.J. The ERK mitogen-activated protein kinase signaling network: The final frontier in RAS signal transduction. Biochem. Soc. Trans. 2021;49:253–267. doi: 10.1042/BST20200507. [DOI] [PubMed] [Google Scholar]
- 137.Kummer L., Parizek P., Rube P., Millgramm B., Prinz A., Mittl P.R., Kaufholz M., Zimmermann B., Herberg F.W., Plückthun A. Structural and functional analysis of phosphorylation-specific binders of the kinase ERK from designed ankyrin repeat protein libraries. Proc. Natl. Acad. Sci. USA. 2012;109:E2248–E2257. doi: 10.1073/pnas.1205399109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sabapathy K., Lane D.P. Understanding p53 functions through p53 antibodies. J. Mol. Cell. Biol. 2019;11:317–329. doi: 10.1093/jmcb/mjz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Caron de Fromentel C., Gruel N., Venot C., Debussche L., Conseiller E., Dureuil C., Teillaud J.L., Tocque B., Bracco L. Restoration of transcriptional activity of p53 mutants in human tumour cells by intracellular expression of anti-p53 single chain Fv fragments. Oncogene. 1999;18:551–557. doi: 10.1038/sj.onc.1202338. [DOI] [PubMed] [Google Scholar]
- 140.Weisbart R.H., Hansen J.E., Chan G., Wakelin R., Chang S.S., Heinze E., Miller C.W., Koeffler P.H., Yang F., Cole G.M., et al. Antibody-mediated transduction of p53 selectively kills cancer cells. Int. J. Oncol. 2004;25:1867–1873. doi: 10.3892/ijo.25.6.1867. [DOI] [PubMed] [Google Scholar]
- 141.Weisbart R.H., Wakelin R., Chan G., Miller C.W., Koeffler P.H. Construction and expression of a bispecific single-chain antibody that penetrates mutant p53 colon cancer cells and binds p53. Int. J. Oncol. 2004;25:1113–1118. [PubMed] [Google Scholar]
- 142.Tal P., Eizenberger S., Cohen E., Goldfinger N., Pietrokovski S., Oren M., Rotter V. Cancer therapeutic approach based on conformational stabilization of mutant p53 protein by small peptides. Oncotarget. 2016;7:11817–11837. doi: 10.18632/oncotarget.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hosein A.N., Dougan S.K., Aguirre A.J., Maitra A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer. 2022;3:272–286. doi: 10.1038/s43018-022-00349-2. [DOI] [PubMed] [Google Scholar]
- 144.Sunami Y., Boker V., Kleeff J. Targeting and Reprograming Cancer-Associated Fibroblasts and the Tumor Microenvironment in Pancreatic Cancer. Cancers. 2021;13:697. doi: 10.3390/cancers13040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., Kadel E.E., III, Koeppen H., Astarita J.L., Cubas R., et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rouanet M., Lebrin M., Gross F., Bournet B., Cordelier P., Buscail L. Gene Therapy for Pancreatic Cancer: Specificity, Issues and Hopes. Int. J. Mol. Sci. 2017;18:1231. doi: 10.3390/ijms18061231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 148.Bery N., Bataille C.J., Russell A., Hayes A., Raynaud F., Milhas S., Anand S., Tulmin H., Miller A., Rabbitts T.H. A cell-based screening method using an intracellular antibody for discovering small molecules targeting the translocation protein LMO2. Sci. Adv. 2021;7:eabg1950. doi: 10.1126/sciadv.abg1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bery N., Cruz-Migoni A., Bataille C.J., Quevedo C.E., Tulmin H., Miller A., Russell A., Phillips S.E., Carr S.B., Rabbitts T.H. BRET-based RAS biosensors that show a novel small molecule is an inhibitor of RAS-effector protein-protein interactions. eLife. 2018;7:e37122. doi: 10.7554/eLife.37122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bery N., Rabbitts T. A cell-based screening method using an intracellular antibody for discovering small molecules targeting hard-to-drug proteins. Bio-Protocol. 2022;12:e4324. doi: 10.21769/BioProtoc.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Canning P., Bataille C., Bery N., Milhas S., Hayes A., Raynaud F., Miller A., Rabbitts T. Competitive SPR using an intracellular anti-LMO2 antibody identifies novel LMO2-interacting compounds. J. Immunol. Methods. 2021;494:113051. doi: 10.1016/j.jim.2021.113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Quevedo C.E., Cruz-Migoni A., Bery N., Miller A., Tanaka T., Petch D., Bataille C.J.R., Lee L.Y.W., Fallon P.S., Tulmin H., et al. Small molecule inhibitors of RAS-effector protein interactions derived using an intracellular antibody fragment. Nat. Commun. 2018;9:3169. doi: 10.1038/s41467-018-05707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bery N., Rabbitts T.H. Bioluminescence Resonance Energy Transfer 2 (BRET2)-Based RAS Biosensors to Characterize RAS Inhibitors. Curr. Protoc. Cell Biol. 2018;83:e83. doi: 10.1002/cpcb.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.