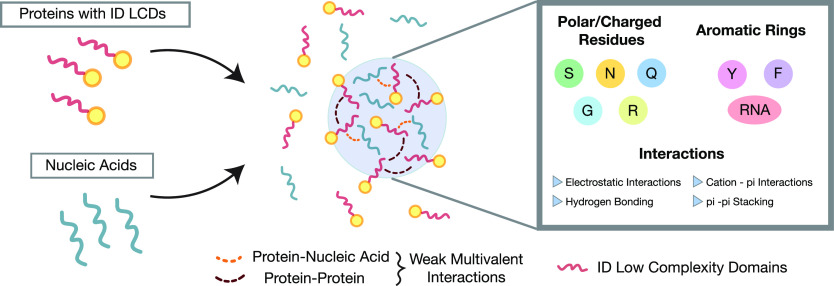
Figure 2.
Biochemical interactions driving LLPS. Proteins containing Intrinsically Disordered Low Complexity Domains (ID LCDs) and nucleic acids are prone to phase separate into membraneless condensates. The drivers for such behavior are weak multivalent interactions, e.g., between proteins or proteins and nucleic acids, and involve polar/charged residues and aromatic rings in the protein residues and RNA. Protein–RNA interactions are typically electrostatic attractions and cation−π interactions. Protein residues typically interact through electrostatic interactions, hydrogen bonding, cation−π, and π–π stacking.