Abstract
Medicinal herbs have been a part of human medicine for thousands of years. The herb–drug interaction is an extension of drug–drug interaction, in which the consumptions of herbs cause alterations in the metabolism of drugs the patients happen to take at the same time. The pregnane X receptor (PXR) has been established as one of the most important transcriptional factors that regulate the expression of phase I enzymes, phase II enzymes, and drug transporters in the xenobiotic responses. Since its initial discovery, PXR has been implicated in multiple herb–drug interactions that can lead to alterations of the drug’s pharmacokinetic properties and cause fluctuating therapeutic efficacies, possibly leading to complications. Regions of the world that heavily incorporate herbalism into their primary health care and people turning to alternative medicines as a personal choice could be at risk for adverse reactions or unintended results from these interactions. This article is intended to highlight our understanding of the PXR-mediated herb–drug interactions.
Keywords: Drug metabolism, Herb-drug interaction, PXR, St. John’s Wort, Xenobiotics
1. Discovery and early characterization of PXR as a xenobiotic receptor
Humans and other mammals are exposed to numerous xenobiotics every day either intentionally or unintentionally through food, water, air, or any other type of environmental exposure. Within the natural products realm of xenobiotics, there exists a seemingly limitless array of chemical entities that could hold the potential for pushing our understanding of currently puzzling diseases to the edge of the scientific frontier. These chemical entities from natural products, or phytochemicals, can become incorporated in the already vast and complex biochemical nature of the human body, and can lead to either intended benefit or unintended harm. The body’s innate ability to sense, react to, and act upon these foreign substances is a remarkable feat that ensures efficient metabolism/detoxification and the restoration of homeostasis.
The cascade of transcription and expression of drug metabolizing enzymes and transporters upon exposure to xenobiotics has been traced to the nuclear receptor pregnane X receptor (PXR). In 1995, Phil Guzelian’s laboratory discovered the existence a novel element in the CYP3A gene promoter that links the glucocorticoid signaling and subsequent CYP3A1 gene activation, which disproved the idea that direct binding of glucocorticoids such as dexamethasone (DEX) to the glucocorticoid receptor (GR) was the mechanism of CYP3A1 gene activation [1]. Two areas of conserved rodent DNA sequence, referred to as “footprints”, within the CYP3A gene promoter lacked the typical glucocorticoid response element (GRE), which further supported the existence of a novel element that is bound by a yet to be defined cellular factor. One year later, Phil Guzelian’s laboratory further proposed that the presence of unique “cellular factors” in each species, instead of allelic heterogeneity in the CYP3A gene itself, accounts for the well-known species specific induction of CYP3A enzymes by the same xenobiotics, such as rifampicin (RIF) and pregnenolone-16α-carbonitrile (PCN) [2]. Two years later, in 1998, PXR was first cloned in the laboratories of Steve Kliewer and Ron Evans utilizing cDNA libraries. PXR was demonstrated to be a novel nuclear receptor activated by endogenous and synthetic steroids and be present in highly metabolic tissues such as the liver and intestines [3,4]. The Kliewer group termed the nuclear receptor PXR since it was activated by the 21-carbon pregnanes, while the Evan’s group termed it steroid and xenobiotic receptor (SXR) due to its activation by natural and synthetic steroid compounds and xenobiotics [3,4]. These findings ultimately set the stage for the discovery of the in vivo functionality of this nuclear receptor that sits at the heart of the xenobiotic response of enzymes and transporters. The DNA “footprints” in the promoter region of CYP3A that were discovered by the Guzelian group contain a PXR response element, which was bound to by the “cellular factor” PXR. This correlation therefore classifies CYP3A as a direct target gene of PXR [5]. This formed speculation of a connection between PXR and drug metabolizing enzyme induction involved in the drug response in vivo. In 2000, the Evans first reported the creation and characterization of the PXR knockout mice, in which the induction of CYP3A by PCN and DEX was completely abolished [6], and these results were independently verified in another strain of PXR knockout mice created in the Kliewer lab [7].
There is a considerable homology in the DNA binding-domain (DBD) of PXR between the human and mouse PXR. This conserved portion of the DBD allows PXR to share promoter binding sites in the CYP3A gene promoters of either the human or rodent origin. In a murine model, disruption of the N-terminal zinc finger portion of the DBD resulted in a truncated and inactive protein, unable to bind to DNA [7]. Homology in the C-terminal ligand binding-domain (LBD) was found to be much less between humans and mice, which postulates the idea of specificity of ligand recognition by PXR in different species [5]. Crystal structure studies of the LBD revealed a more in-depth reasoning for the diverse, yet distinct, ligand binding to PXR [8]. The large and rather unique LBD binding pocket is what allows PXR to bind to a diverse array of ligands, while other traditional nuclear receptors tend to have a more rigid specificity for their ligands [8]. The pocket itself is spherical, hydrophobic, and flexible, which are all characteristics that would be expected for a promiscuous receptor [8]. Within the large hydrophobic portions of the pocket, there exist a small number of polar head residues [8]. Changes within these polar residues can lead to variations in responsiveness to different xenobiotics. This ligand pocket variability supports the idea of species specific forms of PXR and consequently, different xenobiotic responses [8]. Cell transfection and mouse transgenic studies have functionally demonstrated that the species origin of PXR, rather than the structure of the promoter regions, dictates the response to xenobiotics [6]. As a direct result of this finding, “humanized” mice have been created by genetically replacing the mouse PXR (mPXR) with the human PXR (hPXR) [6]. The humanized mice were able to display a more human representative drug response profile rather than a mouse representative drug response profile [6]. The creation of “humanized” PXR mice is a significant step forward in creating a standard model that can be used to test drug–drug interactions, toxicity, and herb–drug interactions in order to create overall safer drugs [6]. This new model also gives us the opportunity to be able to observe potentially harmful interactions between herbal medicines and prescription medicines before they can occur in human patients.
Previous beliefs that PXR was solely in charge of the regulation of phase 1 cytochrome P450 enzymes have been debunked, because emerging evidence has shown that PXR also plays an essential role in the regulation of phase II drug metabolizing enzymes and drug transporters [5]. As of 2009, PXR target genes include phase I cytochrome P450’s (CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A1, CYP3A4, CYP3A5, CYP3A7, CYP4F12, CYP24, and CYP27A1), phase II uridine diphosphate (UDP)-glucuronosyltransferases (UGT1A1, UGT1A3, UGT1A4, UGT1A6, and UGT1A9), sulfotransferases (Sult2a1), glutathione S-transferases (Gsta2, GSTA4), and carboxylesterases (8, 9, 16), and phase III P-glycoprotein (MDR1/ABCB1), multidrug resistance-associated protein 1 (Abcc1), multidrug resistance-associated protein 2 (Abcc2), multidrug resistance-associated protein 3 (Abcc3), and organic anion transporting polypeptide 2 (OATP2) [9,10]. As a result, PXR has since been defined as a master regulator of the xenobiotic response [5].
2. Herb–drug interactions
Medicinal herbs have been a part of human medicine for the last 5000 years and continue to be increasingly involved in modern medicine of the 21st century [11]. An herb is defined as being any type of plant or plant product from the tip of the plant down to the roots in the earth, including and leaves, flowers, and seeds [11]. Herbs contain various phytochemicals, but the proportions of these chemicals can vary substantially from plant to plant. This natural inconsistency lends into the great complexity of studying safety and efficacy of these natural products. In 2007, is has been reported that 40% of adults in America used some form of complementary and alternative medicine, 17.7% of which were natural products [12]. A third of Americans who consume an herbal product concomitantly consume other oral products [13]. On a larger scale, the WHO has reported 70% of the world’s population uses some form of alternative medicine [14]. This becomes especially concerning when it comes to drugs such as chemotherapeutics and immunosuppressive agents because they have a narrow therapeutic index and fluctuations in bioavailability due to enzyme/transporter inhibition or induction can either lead to toxicity by down or loss of therapeutic efficacy [13,15]. Complementary and alternative medicine has been increasing in popularity and usage in western medicine and all over the world [16]. As of July 1, 2017, traditional Chinese medicine, which includes the use of herbal supplements, has been given a much larger role in the country’s medical system and puts it on an importance level comparable with western medicine [17]. Tu Youyou’s discovery of artemisinin as a novel treatment for malaria earned her the 2015 Nobel Prize, perhaps further facilitating the acceptance of TCM into the nation’s healthcare system [17]. The one largest misconception by the public with regards to natural products is the perceived notion that “natural” equals “safe”. It is crucial to understand that natural products can be marketed and sold to consumers with little demonstration of safety of efficacy [11].
Among the herbs, St. John’s Wort (Hypericum perforatum) has become the most studied natural product in the world by amassing over 2000 peer reviewed articles [18]. It has become popular globally for its efficacy as an antidepressant [18]. It has also taken center stage in herb–drug interaction research due to its pharmacokinetic actions on drug metabolizing enzymes (Phase I and II) and efflux/uptake transporters [13]. The US Food and Drug Administration has labeled St. John’s Wort as a strong inducer of CYP 450 enzymes and P-gp transport, which, by definition, can reduce the AUC of a CYP 450 substrate by up to 80% [19]. St. John’s Wort has been demonstrated to have clinically relevant pharmacokinetic effects, leading to decreased plasma bioavailability of HIV protease inhibitors (such as Ritonavir and Indinavir), antidepressants, antihypertensives, cardiovascular medicines, blood pressure medicines, bronchodilators, immunosuppressants, sedatives, and steroid hormones such as oral contraceptives, to name a few [16]. In 2003, it was reported that a 36-year-old woman became pregnant while taking the oral contraceptive Valette® in addition to self-medicating with St. John’s Wort after a failed suicide attempt in 1995 [20]. Investigations into this herb–drug interaction began increasing in number with the first controlled clinical trial taking place in 2003. The trial concluded that irregular bleeding episodes along with decreased serum concentration of oral contraceptive taken together with St. John’s Wort put women at higher risk of unwanted pregnancies, or as some called them, “miracle babies” [21]. In addition to oral contraceptives, St. John’s Wort co-administered with warfarin has shown to reduce its ability to increase prothrombin time and puts patients at increased risk of an adverse cardiovascular event [22]. Patients diagnosed with HIV-1 who take Indinavir as their protease inhibitor in combination with St. John’s Wort are at significant risk of treatment failure or developing retroviral resistance [23]. In another study, 35 patients received liver or kidney transplants and along with taking their prescribed cyclosporine, self-medicated with St. John’s Wort resulting in their therapeutic plasma level being reduced by almost 50% and causing 2 serious organ rejection scenarios [24]. This type of immunosuppression drug–herb interaction has also been documented in a heart transplant patient who was self-medicating with St. John’s Wort for mild depression prior to the surgical procedure [25]. Cancer patients taking Imatinib for their treatment are also subject to significant plasma level decrease of the drugs taken along with St. John’s Wort [26]. An additional study involving the herb and anti-fungal agent voriconazole highlights the more complicated nature of this type of interaction. Long-term interaction between St. John’s Wort and voriconazole decreased the drug’s bioavailability while paradoxically short-term interaction increased the drug’s bioavailability [27]. The cases described above are only representative of a single herb’s impact on multiple medical fronts.
There are a plethora of other herbs, some common and some unusual, that alter the pharmacokinetics of concomitant drugs, but require additional evidence to establish causation. Echinacea purpurea is an herb that has gained popularity in its observed ability to alleviate secondary symptoms of HIV in antiretroviral therapy, but has been shown to up-regulate CYP1A2, CYP3A4, and MDR1 (P-gp) in vitro which could ultimately affect a prescription drug’s performance [28]. To put the issue of herb–drug interactions into a more relatable perspective, we can look at herbs that are taken not for their medicinal properties, but for their appetizing aromatic properties and their ability to inject flavors into our snacks, meals or beverages. Black pepper, cayenne pepper, garlic, ginger, ginkgo balboa, ginseng, grapefruit juice, liquorice, and pomegranate juice are all being investigated to determine their risk of causing an adverse event when taken with medications [18,29,30]. The variation in the results of these herbs with conventional medicine warrants further research into the mechanisms and causalities of potential adverse events. It is critical to widen our knowledge base in this area in order to prevent, or at least have some degree of control over, the fate of herb–drug interactions.
3. PXR as a mediator of herb–drug interaction
Although the herb–drug interactions have been recognized and extensively studied for many years, the underlying mechanism by which herbs trigger herb–drug interaction has long been elusive. The discovery of PXR in 1998 offered an exciting opportunity to decipher the herb–drug interactions. Indeed, structural analysis revealed that the binding pocket of PXR exhibits a “one-size-fits-all” type of structure [31]. It seems reasonable to speculate that many xenobiotic chemicals, including those present in the herbs, can be recognized by PXR and consequently trigger a PXR-mediated regulation of drug metabolizing enzymes, which can be the molecular basis of drug–herb interactions.
The first herb–PXR interaction that was uncovered was the discovery of St. John’s Wort extract and its constituent compound hyperforin acting as PXR agonists, leading to increased hepatic drug metabolism [32]. In 2000, the group at Glaxo Wellcome, led by Steve Kliewer, demonstrated a direct binding of hyperforin to the LBD of PXR [32]. The EC50 of induction of CYP3A in primary human hepatocytes was measured at 23 nM, that is at ~94% less than the of the concentration of blood plasma after a standard 3 × 300 mg dose regimen, which really shows how potent of a PXR activator and enzyme inducer this really is [32]. This degree of inducibility can cause increased clearance of a systemically available drug that just also happens to be a substrate of CYP3A.
Since the discovery of St. John’s Wort as a PXR agonist, a whole host of herbs, including gugulipids, kava kava, Coleus forskohlii, hypoxis, sutherlandia, qing hao, gan cao and quiercitin, have been found to interact with PXR in some fashion to trigger the up-regulation of enzymes and transporters to expedite their own metabolism and subsequently the metabolism of concomitant drugs that are substrates for the same enzymes and transporters [33]. These herb–drug interactions are starting to build evidence and starting to become more understood, but not quite to the degree of St. John’s Wort. Herb–drug interactions through PXR activation are important biochemical mechanisms that should be investigated with every new drug that emerges onto the market to preserve the safety of those individuals who will be taking it. The herb–PXR interaction can be evaluated by using cell culture models and/or in vivo animal models. These include the PXR transfection and reporter gene assay and the PXR “humanized mice”.
4. Does the induction of PXR always correlate to an increase in drug metabolism?
PXR induction has been shown to cause adverse pharmacokinetic reactions such as increased drug metabolism leading to sub-therapeutic concentrations of important drugs. An outstanding question is: Does the induction of PXR always correlate to an increase in drug metabolism? Studies showed that some xenobiotics display a dual nature of acting as a PXR activator/CYP enzyme inducer and as a CYP enzyme inhibitor. The enzyme inhibitory effects of xenobiotics can be categorized as either reversible inhibition or irreversible inhibition, which is commonly referred to as mechanism based inhibition [34]. Irreversible inhibition essentially inactivates the enzyme through irreversible binding and in vivo, the only way for the CYP enzyme ability to be restored is to synthesize new enzymes [34]. This type of inhibition has drawn clinical interest due to the extended time of which CYP enzymes are unable to metabolize their substrates due to the relatively slower process of protein biosynthesis [34]. In the event that a xenobiotic compound can function as both a PXR activator and enzyme/transporter inhibitor, the final outcome depends on the relative strength of these two opposing actions.
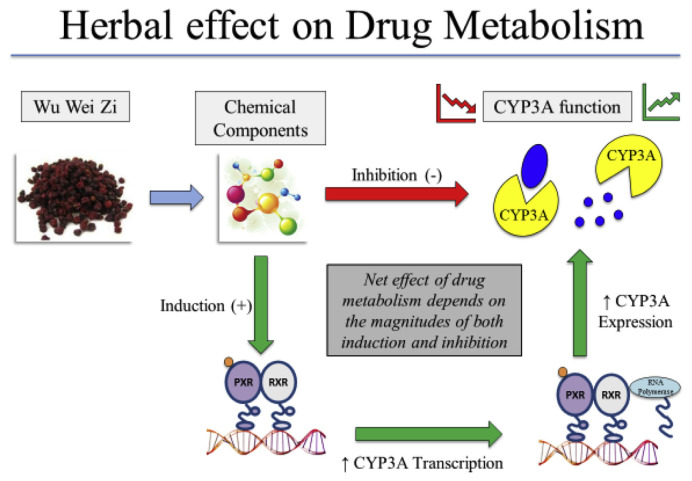
The traditional Chinese herbal medicine Wu Wei Zi (WWZ, or Schisandra Sphenanthera) provides a good example that an herb may have a complex effect on drug metabolism as summarized in Fig. 1. WWZ has been shown to activate PXR and induce CYP enzymes. Schisandrol B, Schisandrins A and Schisandrins B were identified as the PXR-activating constituents isolated from WWZ that showed similar potency and efficacy as RIF [35]. However, WWZ extracts have been used as a drug-sparing agent to decrease drug metabolism of immunosuppressants such as Tacromilus in a pre-clinical rat model [36]. The drug sparing effect of WWZ was reasoned to be due to the inhibitory effect of WWZ on the activities of both CYP3A enzyme and P-gp, the enzymes and transporter that play an important role in the disposition of Tacromilus. Wu Wei Zi taken concomitantly with Tacromilus allows the herb to exhibit its inhibitory effects on CYP3A enzymes and P-gp transporter proteins resulting in an improved systemic bioavailability and a higher concentration of drug being able to survive first pass metabolism [36]. Treatment with WWZ has also been shown to improve the bioavailability of cyclosporine A and paclitaxel in animal models [34]. The overall drug sparing effect of WWZ suggests that the enzyme and transporter inhibitory effect of WWZ dominates the PXR activating and enzyme inducing activity of this herbal.
Fig. 1.
Summary of the effect of Wu Wei Zi on drug metabolism. The overall net effect of an herb–drug interaction depends on the proportion of induction and inhibition triggered by constitutive phytochemicals contained in the herb. Phytochemical contents of herbs are generally susceptible to great variability in nature.
Other factors, besides the inhibition of enzyme and transporter activity, have been discovered to play a role in determining whether PXR induction will lead to increased drug metabolism. In vitro studies investigating a drug’s ability to induce PXR were not entirely reflective of observed in vivo drug concentrations that caused some drugs to be labeled as inducers when in fact they showed no induction in vivo [34]. Intracellular concentrations of the drugs in vivo are also more susceptible to fluctuation due to the presence of transporters which presents the case that drug concentrations in vitro may not be the same observed in vivo [34]. Another complexity is the species specificity of PXR activation and enzyme induction, meaning that cautions need to apply when extrapolating preclinical rodent model data to humans.
5. Summary and perspectives
After 22 years of the discovery of PXR, we have gained a significant understanding of its importance in the metabolism and disposition of xenobiotic and endogenous chemicals. From being initially regarded as strictly a phase I CYP 450 enzyme inducer to an inducer of phases I–III, this receptor continues to demonstrate complexity and variability. Herb–drug interaction studies seem to be gaining traction in the realm of pharmaceutical research and with good reason. Natural products, by nature, are more complex than what we can ever hope to achieve synthetically in the lab. One-third to one-half of today’s pharmaceutical company’s products are being derived from nature, more specifically from plants [9]. There are approximately 20,000 herbal supplements in existence, as well as in use, that have little pharmacokinetic, pharmacodynamic, or safety data [9]. The key for future research is to study these herb–PXR interactions while taking into consideration the delicate balance between induction and inhibition, in vivo concentrations of drugs, and understanding that rodent models may not fully represent the intricate and variable nature of the human body. Future research could also include identifying of agonists of PXR, finding the pharmacophore structure, determining whether PXR induction or inhibition would be of most benefit, and synthetically modifying the agonist or antagonist in a way that produces the most beneficial therapeutic effect, essentially giving rise to a type of precision medicine. This type of research has already gotten off the ground in the case where 820 ingredients from 421 herbs were screened as agonists of PXR, building a pharmacophore model, and using docking strategies to identify and rank potential PXR agonists [37]. Although this article focuses on the discussion of PXR, it is important to emphasize that the constitutive androstane receptor (CAR) is a sister xenobiotic receptor of PXR. CAR share many of the functions of PXR in sensing herb medicines and in regulating the expression of drug metabolizing enzymes and transporters [38, 39].
REFERENCES
- 1. Quattrochi LC, Mills AS, Barwick JL, Yockey CB, Guzelian PS. A novel cis-acting element in a liver cytochrome P450 3A gene confers synergistic induction by glucocorticoids plus antiglucocorticoids. J Biol Chem. 1995;270:28917–23. doi: 10.1074/jbc.270.48.28917. [DOI] [PubMed] [Google Scholar]
- 2. Barwick JL, Quattrochi LC, Mills AS, Potenza C, Tukey RH, Guzelian PS. Trans-species gene transfer for analysis of glucocorticoid-inducible transcriptional activation of transiently expressed human CYP3A4 and rabbit CYP3A6 in primary cultures of adult rat and rabbit hepatocytes. Mol Pharmacol. 1996;50:10–6. [PubMed] [Google Scholar]
- 3. Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 4. Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado Jr J, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan J, Xie W. A brief history of the discovery of PXR and CAR as xenobiotic receptors. Acta Pharm Sin B. 2016;6(5):450–2. doi: 10.1016/j.apsb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–8. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 7. Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–33. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 9. Mandelkar S, Hong JL, Kong ANT. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr Drug Metabol. 2006;7:661–75. doi: 10.2174/138920006778017795. [DOI] [PubMed] [Google Scholar]
- 10. Chang TK. Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 2009;11:590–601. doi: 10.1208/s12248-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J General Intern Med. 2008;23(6):854–9. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes PM, Bloom B, Nahin RL. National health statistics reports no 12. Hyattsville, MD: National Center for Health Statistics; 2008. Complementary and alternative medicine use among adults and children: United States, 2007. [PubMed] [Google Scholar]
- 13. Mouly S, Llorent-Linares C, Sellier PO, Sene D, Bergmann JF. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacol Res. 2017;118:82–92. doi: 10.1016/j.phrs.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 14. Fasinu P, Bouic P, Rosenkranz B. An overview of the evidence and mechanisms of herb-drug interactions. Front Pharmacol. 2012;3(69):1–19. doi: 10.3389/fphar.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meijerman I, Beijnen JH, Schellens J. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncol. 2006;11:742–52. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- 16. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Tianchen H. China adopts law on TCM to boost medical practices. CGTN; Dec 26, 2016. news.cgtn.com/news/3d6b544f7955544d/share_p.html . [Google Scholar]
- 18. Gurley BJ, Fifer EK, Gardner Z. Pharmacokinetic herb-drug interactions (part 2): drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012;78:1490–514. doi: 10.1055/s-0031-1298331. [DOI] [PubMed] [Google Scholar]
- 19. Berry-Bibee E, Kim MJ, Tepper N, Riley H, Curtis K. Co-administration of St. John’s Wort and hormonal contraceptives: a systematic review. Contraception. 2016;94(6):668–77. doi: 10.1016/j.contraception.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwarz U, Büschel B, Kirch W. Unwanted pregnancy on self-medication with St. John’s wort despite hormonal contraception. Br J Clin Pharmacol. 2003;55(1):112–3. doi: 10.1046/j.1365-2125.2003.t01-1-01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfrunder A, Schiesser M, Gerber S, Haschke M, Bitzer J, Drewe J. Interaction of St. John’s wort with low-dose oral contraceptive therapy: a randomized controlled trial. Br J Clin Pharmacol. 2003;56(6):683–90. doi: 10.1046/j.1365-2125.2003.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55(6):515–25. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s wort. Lancet. 2000;355:547–8. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 24. Ernst E. St. John’s Wort supplements endanger the success of organ transplantation. Arch Surg. 2002;137:316–9. doi: 10.1001/archsurg.137.3.316. [DOI] [PubMed] [Google Scholar]
- 25. Ruschitzka F, Meier PJ, Turina M, Luscher TF, Noll G. Acute heart transplantation rejection due to St. John’s wort. Lancet. 2000;355:548–9. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 26. Frye R, Fitzgerald S, Lagattuta T, Hruska M, Egorin M. Effect of St. John’s Wort on imatinib mesylate pharmacokinetics. Clin Pharmacol Therapeut. 2004;76:323–9. doi: 10.1016/j.clpt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 27. Xie HG, Kim R. St. John’s wort-associated drug interactions: short-term inhibition and long-term induction? Clin Pharmacol Therapeut. 2005;78(1):19–24. doi: 10.1016/j.clpt.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28. Awortwe C, Manda V, Avonto C, Khan S, Khan I, Walker L, et al. Echinacea purpurea up-regulates CYP1A2, CYP3A4 and MDR1 gene expression by activation of pregnane X receptor pathway. Xenobiotica. 2015;45(3):218–29. doi: 10.3109/00498254.2014.973930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adriane Fugh-Berman. Herb-Drug interactions. Lancet. 2000;355(9198):134–8. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 30. Prakash C, Zuniga B, Song CS, Jiang S, Cropper J, Park S, et al. Nuclear receptors in drug metabolism, drug response and drug interactions. Nucl Recept Res. 2015;2 doi: 10.11131/2015/101178. pii:101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kliewer S. Nuclear Receptor PXR: discovery of a pharmaceutical anti-target. J Clin Investig. 2015;125(4):1388–9. doi: 10.1172/JCI81244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staudinger J, Ding X, Lichti K. Pregnane X receptor and natural products: beyond drug-drug interactions. Expert Opin Drug Metab Toxicol. 2006;2(6):847–57. doi: 10.1517/17425255.2.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei Y, Tang C, Sant V, Li S, Poloyac S, Xie W. A molecular aspect in the regulation of drug metabolism: does PXR-induced enzyme expression always lead to functional changes in drug metabolism? Curr Pharmacol Rep. 2016;2:187–92. doi: 10.1007/s40495-016-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Therapeut. 2006;316(3):1369–77. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 36. Qin XL, Bi HC, Wang XD, Li JL, Wang Y, Xue XP, et al. Mechanistic understanding of the different effects of Wuzhi Tablet (Schisandra sphenanthera extract) on the absorption and first-pass intestinal and hepatic metabolism of Tacrolimus (FK506) Int J Pharm. 2010;389(1–2):114–21. doi: 10.1016/j.ijpharm.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 37. Cui Z, Kang H, Tang K, Liu Q, Cao Z, Zhu R. Screening ingredients from herbs against pregnane X receptor in the study of inductive herb-drug interactions: combining pharmacophore and docking-based rank aggregation. Biomed Res Int. 2015;2015:657159. doi: 10.1155/2015/657159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407(6806):920–3. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 39. Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Investig. 2004;113(1):137–43. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]