Abstract
Indican (indoxyl-β-D-glucoside) is present in several Chinese herbs e.g. Isatis indigotica, Polygonum tinctorium and Polygonum perfoliatum. The major metabolite of indican was indoxyl sulfate (IS), an uremic toxin which was a known substrate/inhibitor of organic anion transporter (OAT) 1, OAT 3 and multidrug resistance-associated protein (MRP) 4. Methotrexate (MTX), an important immunosuppressant with narrow therapeutic window, is a substrate of OAT 1, 2, 3, 4 and MRP 1, 2, 3, 4. We hypothesized that IS, the major metabolite of oral indican, might inhibit the renal excretion of MTX mediated by OAT 1, OAT 3 and MRP 4. Therefore, this study investigated the effect of oral indican on the pharmacokinetics of MTX. Rats were orally given MTX with and without indican (20.0 and 40.0 mg/kg) in a parallel design. The serum MTX concentration was determined by a fluorescence polarization immunoassay. For mechanism clarification, phenolsulfonphthalein (PSP, 5.0 mg/kg), a probe substrate of OAT 1, OAT 3, MRP 2 and MRP 4, was intravenously given to rats with and without a intravenous bolus of IS (10.0 mg/kg) to measure the effect of IS on the elimination of PSP. The results indicated that 20.0 and 40.0 mg/kg of oral indican significantly increased the area under concentration–time curve0-t (AUC0-t) of MTX by 231% and 259%, prolonged the mean residence time (MRT) by 223% and 204%, respectively. Furthermore, intravenous IS significantly increased the AUC0-t of PSP by 204% and decreased the Cl by 68%. In conclusion, oral indican increased the systemic exposure and MRT of MTX through inhibition on multiple anion transporters including OAT 1, OAT 3 and MRP 4 by the major metabolite IS.
Keywords: Indican, Indoxyl sulfate, Methotrexate, Anion transporters, Pharmacokinetics
1. Introduction
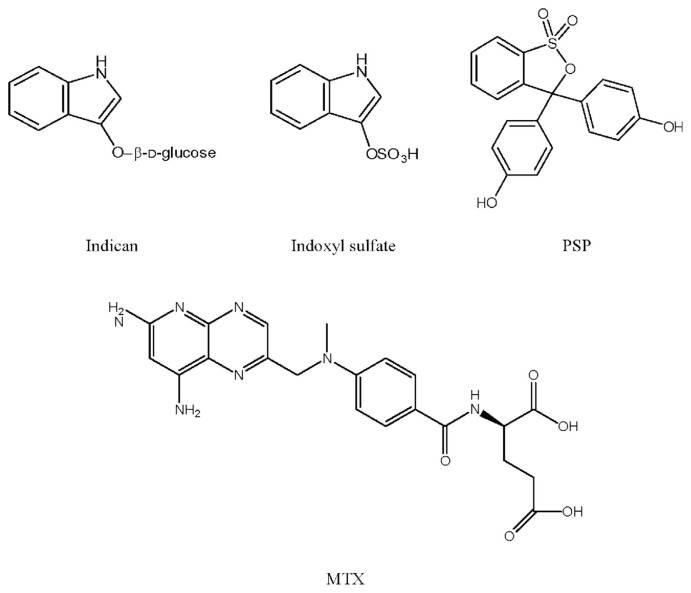
Indican (indoxyl-β-D-glucoside, chemical structure shown in Fig. 1) is a constituent of several Chinese herbs such as Isatis indigotica, Polygonum tinctorium and Polygonum perfoliatum, which are used to treat colds, fever and influenza in clinical Chinese medicine [1,2]. Pharmacological studies of these herbs have reported numerous beneficial effects such as anti-inflammatory [3], antipyretic [3], antiviral [2,4], antimicrobial [5] and anticancer activities [4,6].
Fig. 1.
Structures of indican, indoxyl sulfate, phenolsulfonphthalein (PSP) and methotrexate (MTX).
In pharmacokinetic aspect, indican was mainly metabolized to indoxyl sulfate (IS) [7], which was a well known endogenous uremic toxin derived from tryptophan [7–9] and also associated with the progression of cardiovascular diseases [10–12]. Being a strong acid, IS is completely ionized in bloodstream and thus unable to permeate cell membrane via passive diffusion [13]. Recent studies reported that IS was a substrate/inhibitor of organic anion transporters (OAT) such as OAT 1, OAT 3 and multidrug resistance-associated protein (MRP) 4 [14,15].
OATs and MRPs have been well recognized as important transporters for anions, which were responsible for the uptake and efflux transports of numerous acidic compounds at various organs [16,17]. Methotrexate (MTX), a dicarboxylic acid, is a substrate of numerous anion transporters such as OAT 1, 2, 3, 4 and MRP 1, 2, 3, 4 [18,19]. In clinical practice, MTX is commonly used for the treatment of certain neoplastic diseases, rheumatic arthritis and psoriasis, but with narrow therapeutic window. More than 80% of MTX was excreted via urine by OATs- and MRPs-mediated transports [20]. The serum levels of MTX should be carefully monitored because of the accompanied adverse drug reactions, including gastrointestinal problems, central nervous system symptoms, pulmonary damage, hepatotoxicity and nephrotoxicity [21–23].
We herein hypothesized that IS, the major metabolite of oral indican, might inhibit the renal excretion of MTX mediated by the anion transporters including OAT 1, OAT 3 or MRP 4. Therefore, this study investigated the effect of oral indican on the pharmacokinetics of MTX, a probe substrate of OAT 1, 2, 3, 4 and MRP 1, 2, 3, 4 in rats. Furthermore, in order to verify the proposed mechanism, phenolsulfonphthalein (PSP), a substrate of OAT 1, OAT 3, MRP 2 and MRP 4, was injected intravenously to rats as a probe to measure the effect of intravenous IS, which mimicked the metabolite of oral indican, on its elimination.
2. Materials and methods
2.1. Chemicals
Indican, indoxyl sulfate (IS) and phenolsulfonphthalein (PSP) were obtained from Sigma–Aldrich Chemical Co. (St. Louis, MO, U.S.A.). Methotrexate (MTX) (25.0 mg/mL) was obtained from Wyeth Pharma Gmbh (Wolfratshausen, Germany). MK 571 (purity 98%) was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS), 3-(4′,5′-dimethylthiazol-2′-yl)-2,5-diphenyltetrazolium bromide (MTT) and triton X-100 were supplied by Sigma (St. Louis, MO, USA). Fetal Bovine Serum (FBS) was obtained from Biological Industries Inc. (Kibbutz, Beit Haemek, Israel). Penicillin-Streptomycin-Glutamine, Dulbecco’s Modified Eagle Medium (DMEM), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, Hank’s Buffered Salt Solution (HBSS), 5-chloromethylfluorescein diacetate (CMFDA) and trypsin/EDTA were purchased from Invitrogen (Grand Island, NY, USA). TDx kit of MTX was purchased from Abbott Laboratories (Abbott Park, IL, USA). Other reagents were HPLC grade or reagent grade. Milli-Q plus water (Millipore, Bedford, MA, USA) was used throughout this study.
2.2. Animals
The animal study adhered to “The Guidebook for the Care and Use of Laboratory Animals (2002)” published by the Chinese Society of Animal Science, Taiwan. Male Sprague–Dawley rats were supplied by National Laboratory Animal Center (Taipei, Taiwan) and kept in the animal center of Chinese Medical University (Taichung, Taiwan). The Institutional Animal Care and Use Committee (IACUC), China Medical University approved this animal protocol. All blood samplings were conducted under anesthesia with 2–3% isoflurane to minimize the suffering and distress of rats.
2.3. Effect of oral indican on the pharmacokinetics of oral MTX in rats
2.3.1. Drug administration
Male Sprague–Dawley rats weighing 330–400 g were fasted 12 h before drug administration. MTX (5.0 mg/kg) was given orally with and without 20.0 and 40.0 mg/kg of indican in parallel design. The doses of indican chosen were based on previous quantitation and pharmacological studies of I. indigotica [3,24].
2.3.2. Blood collection
Blood samples (0.4 mL) were withdrawn at 15, 30, 60, 120, 240, 480, 720, 1440, 2160 and 2880 min after MTX dosing. The blood samples were collected in microtubes and centrifuged at 10,000 g for 15 min to obtain the serum. Serum was stored at −20 °C before analysis.
2.3.3. Determination of serum MTX concentration
The serum concentration of MTX was determined by fluorescence polarization immunoassay (FPIA). The assay was calibrated for concentrations from 0 to 1.0 μmol/L and the lower limit of quantitation is 0.01 μmol/L.
2.4. Effect of intravenous IS on the elimination of PSP in rats
2.4.1. Drug administration
Male Sprague–Dawley rats weighing 370–420 g were fasted 12 h before PSP administration. PSP (5.0 mg/kg) was given intravenously with and without intravenous IS (10.0 mg/kg) in a crossover design. One week was allowed for washout.
2.4.2. Blood collection
Blood samples (0.7 mL) were withdrawn at 30, 60, 120, 240, 480, 600, 720 and 1440 min after the intravenous bolus of PSP. The blood samples were centrifuged at 10,000 g for 15 min to obtain serum, which was stored at −20 °C before analysis.
2.4.3. Determination of serum PSP concentration
The serum sample (100 μL) was deproteinized with 300 μL of methanol and basified with 20 μL of 1.0 N NaOH. The concentration of PSP was then determined at 560 nm using ELISA reader [25]. For calibrator preparation, 100 μL of serum was spiked with various concentrations of standards to afford a series of serum standards in the concentration range of 0.39–12.5 μg/mL. The later procedure followed that described above. Calibration curves were plotted by linear regression of optical density against concentrations of PSP.
2.5. Cell line and culture conditions
Madin–Darby canine kidney type II transfected cells with overexpression of MRP 2 (MDCKII-MRP 2) was kindly provided by Prof. Dr. Piet Borst (Netherlands Cancer Institute, Amsterdam, Netherlands). Cells were grown in DMEM medium supplemented with 10% FBS, 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 292 μg/mL of glutamine at 37 °C in a humidified incubator containing 5% CO2. The medium was changed every other day and cells were subcultured when 80%–90% confluency was reached.
2.6. Cell viability assays
The effects of tested drugs on the viability of MDCKII-MRP 2 cells were evaluated by MTT assay [26]. Cells were seeded into a 96-well plate. After overnight incubation, the tested drugs were added into the wells and incubated for 24 h, then 15 μL of MTT (5.0 mg/mL) was added into each well and incubated for additional 3–4 h. During this period, MTT was reduced to formazan crystal by live cells. Acid-SDS (10%) solution was added to dissolve the purple crystal at the end of incubation and the optical density was detected at 570 nm by a microplate reader (BioTex, Highland Park, Winooski, VT, USA).
2.7. Effect of IS on MRP 2-mediated efflux transport
Transport study was conducted to measure the effect of IS on MRP 2 activity [27]. Briefly, MDCKII-MRP 2 cell suspension (4 × 105) was incubated with CMFDA and IS or MK571, a positive MRP 2 inhibitor, at 37 °C for 30 min. After centrifugation, the cell pellet was re-suspended by ice-cold PBS. Subsequently, the intracellular accumulation of glutathione S-methylfluorescein (GSMF), a metabolite of CMFDA and a fluorescent substrate of MRP 2, was determined by a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a standard argon laser for 488 nm excitation and 525 nm bandpass filter.
2.8. Data analysis
The pharmacokinetics of MTX and PSP were analyzed using noncompartment model with the aid of Phoenix WinNonlin (version 6.3, Pharsight Corp., NC, USA). The peak serum concentrations (Cmax) of MTX were obtained from experimental measurement. The areas under the serum concentration–time curve (AUC0-t) were calculated using trapezoidal rule to the last point. One-way ANOVA with Scheffe’s test was used to analyze the differences among three groups. Paired Student’s t-test was used to analyze the differences between two groups.
3. Results
3.1. Effect of oral indican on the pharmacokinetics of oral methotrexate (MTX) in rats
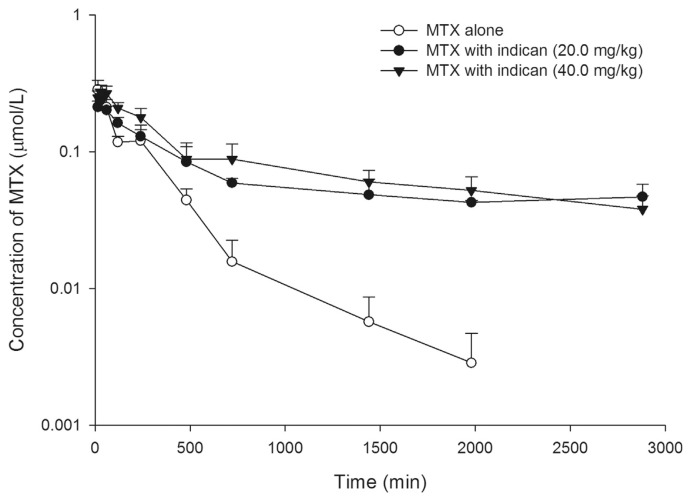
Fig. 2 depicts the serum MTX profiles after oral administration of MTX (5.0 mg/kg) alone and coadministered with 20.0 and 40.0 mg/kg of oral indican. The pharmacokinetic parameters of MTX after three treatments are listed in Table 1. After co-administrations with 20.0 and 40.0 mg/kg of indican, the area under concentration–time curve0-t (AUC0-t) of MTX were significantly increased by 231% and 259%, and mean residence time (MRT) were significantly increased by 223% and 204%, respectively.
Fig. 2.
Mean (±S.E.) serum semi-log concentration–time profiles of methotrexate (MTX) after oral MTX alone (5.0 mg/kg) (○), and coadministrations with 20.0 (●) and 40.0 mg/kg (▼) of indican, revealing that the elimination of MTX was inhibited by both dosages of indican.
Table 1.
Pharmacokinetic parameters of methotrexate (MTX) after oral MTX (5.0 mg/kg) alone and coadministered with 20.0 and 40.0 mg/kg of indican (ID) (n = 5 in each group).
| Parameters | MTX alone | MTX + ID (20.0 mg/kg) | MTX + ID (40.0 mg/kg) |
|---|---|---|---|
| Cmax | 0.3 ± 0.02 | 0.3±0.02 | 0.3±0.03 |
| AUC 0-2880 | 63.6 ± 6.9a | 210.2 ± 28.4b (+231%) | 228.2 ± 39.3 b (+259%) |
| MRT | 315.5 ± 65.5a | 1018.0 ± 54.7b (+223%) | 958.9 ± 93.3b (+204%) |
Data expressed as mean ± S.E.
Means in a row without a common superscript differ (P < 0.05). A mean with superscript “a” was significantly different from a mean with superscript “b”.
Cmax (μmole/L): the peak serum concentration.
AUC0-2880 (μmole•min/L): the area under concentration–time curve to 2880 min.
MRT (min): the mean residence time.
3.2. Effect of indoxyl sulfate (IS) on the pharmacokinetics of intravenous phenolsulfonphthalein (PSP) in rats
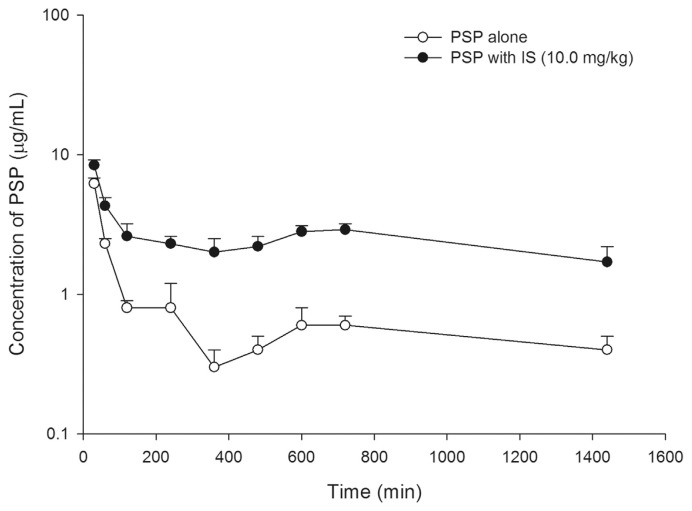
Fig. 3 depicts the serum PSP concentration profiles after an intravenous bolus of PSP (5.0 mg/kg) without and with an intravenous bolus of IS (10.0 mg/kg). The pharmacokinetic parameters of PSP after two treatments are listed in Table 2. When IS was coadministered, the AUC0-t of PSP was significantly increased by 204%, and the clearance (Cl) was significantly decreased by 68%.
Fig. 3.
Mean (±S.E.) serum semi-log concentration–time profiles of phenolsulfonphthalein (PSP) after an intravenous bolus of 5.0 mg/kg PSP without (○) and with 10.0 mg/kg of indoxyl sulfate (IS, ●), revealing that the elimination of PSP was inhibited by IS.
Table 2.
Pharmacokinetic parameters of phenolsulfonphthalein (PSP) after an intravenous bolus of PSP (5.0 mg/kg) alone and with an intravenous bolus of indoxyl sulfate (IS) (10.0 mg/kg) in rats (n = 6 in each group).
| Parameters | PSP alone | PSP + IS | Difference (%) |
|---|---|---|---|
| AUC 0-1440 | 1276.7 ± 118.5 | 3881.4 ± 517.8** | +204% |
| Cl | 3.1 ± 0.4 | 1.0 ± 0.3*** | −68% |
| MRT | 400.0 ± 48.0 | 572.5 ± 23.3** | +43% |
Data expressed as mean ± S.E.
P < 0.01,
P < 0.001 compared with PSP alone.
AUC0-1440 μg•min/mL): the area under concentration–time curve to 1440 min.
Cl (mL/min/kg): the clearance.
MRT (min): the mean residence time.
3.3. Effect of IS on multidrug resistance-associated protein (MRP) 2-mediated efflux transport
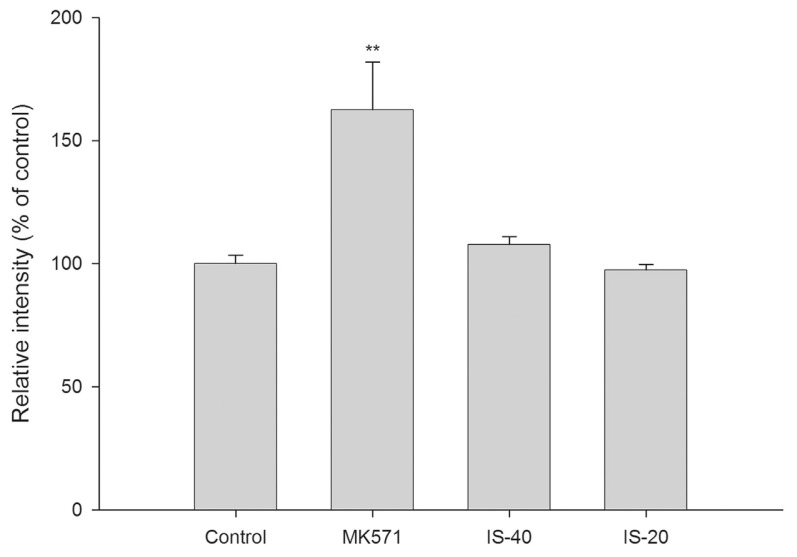
Fig. 4 shows the effect of IS on the intracellular accumulation of glutathione S-methylfluorescein (GSMF), a fluorescent metabolite of 5-chloromethylfluorescein diacetate (CMFDA) and a substrate of MRP 2, in MDCKII-MRP 2 cells. The results indicated that IS did not affect the intracellular accumulation of GSMF. As a positive control of MRP 2 inhibitor, MK 571 (100 μM) increased the intracellular accumulation of GSMF by 63%.
Fig. 4.
Effects of indoxyl sulfate (IS, μM) and MK571 (μM) on the accumulation of glutathione S-methylfluorescein (GSMF), a fluorescent substrate of MRP 2 in MDCKII-MRP 2 cells (**P < 0.01), indicating that IS did not affect the MRP 2-mediated efflux transport of GSMF.
4. Discussion
The results of indican - methotrexate (MTX) interaction study indicated that both 20.0 and 40.0 mg/kg of oral indican significantly increased the systemic exposure and the mean residence time (MRT) of MTX in rats. However, the effect sizes between two dosages of indican were comparable, which could be explained by the nonlinear pharmacokinetics of indican at these doses reported previously [7]. Through observing the serum profiles of MTX, it revealed that the curves during the elimination phase were elevated by both dosages of indican, indicating that the elimination of MTX was inhibited.
It has been known that renal excretion was the major elimination route of MTX [20]. The uptake transports of MTX across the cell membrane of epithelium cells in proximal renal tubule were mediated by anion transporters such as organic anion transporter (OAT) 1, 2, 3, 4, and the efflux transports of MTX were mediated by MRP 1, 2, 3, 4 [18,19]. Therefore, these anion transporters were responsible for the renal excretion of MTX. On other hand, indoxyl sulfate (IS), the major metabolite of indican [7], was also primarily eliminated via kidney via anion transporters such as OAT 1, OAT 3 and MRP 4 [14,28]. Meanwhile, IS was also an inhibitor of OAT 1, OAT 3 and MRP 4 [13,15,29]. Therefore, our results showing that oral indican hampered the elimination of MTX implied that its major metabolite IS decreased the renal excretion of MTX through inhibition on the anion transporters such as OAT 1, OAT 3 or MRP 4.
In order to verify the proposed mechanism, an intravenous bolus of IS mimicking the metabolite of indican was given to rats for measuring the effect on the pharmacokinetics of intravenous phenolsulfonphthalein (PSP), an agent often used as a substrate for the renal organic anion transport system [30,31]. Previous studies have reported that OAT 1 and OAT 3 were responsible for the renal uptake of PSP, while MRP 2 and MRP 4 mediated its renal excretion [31,32]. Therefore, PSP was employed in this study as an in vivo probe substrate of OAT 1, OAT 3, MRP 2 and MRP 4 to verify that IS inhibited these anion transporters in the indican - MTX interaction. As we expected, our results showing that IS significantly increased the systemic exposure and decreased the clearance of PSP indicated that IS hampered the renal excretion of PSP, which could be accounted for by that IS inhibited the uptake transport mediated by OAT 1 and OAT 3 and/or the efflux transport mediated by MRP 2 and MRP 4.
IS was known as an inhibitor of MRP 4 [15]. Whether IS inhibited MRP 2 remained unknown and thus investigated in this study. The results of transport study using MDCKII-MRP 2 cells showed that IS at 20 and 40 μM did not show inhibition on the function of MRP 2. Regarding the relative protein expression levels of MRP 2 and MRP 4 in kidney, MRP 4 was higher than MRP 2 [33]. In addition, the transport of MTX via MRP 4 was with lower Km than MRP 2, indicating MRP 4 has higher affinity for MTX and more easily been saturated [34]. Therefore, the inhibition of IS on MRP 4-mediated efflux transport of MTX could explain the decreased renal excretion of MTX in rats, whereas MRP 2 did not play any role in this indican - MTX interaction.
In regard to OAT 1 and OAT 3, their high protein expression levels in the kidney and their potential roles in the renal excretion of anionic drugs have been extensively studied [16]. IS was known as an inhibitor of OAT 1 and OAT 3 [13,29]. Although OAT 3 showed higher affinity for MTX than OAT 1 [20,35], IS showed greater inhibition on OAT 1 than OAT 3 [13,29]. Taken together, we can infer that IS hampered the renal excretion of MTX through inhibitions on multiple anion transporters including OAT 1 and OAT 3 in addition to MRP 4.
Based on previous pharmacokinetic study [7], indican-containing herbs would result in increased level of IS in blood, especially for patients with chronic kidney disease (CKD), who often had accumulated IS in the body [11,36]. We speculated that for CKD patients, the elevated IS might exert greater inhibition on the renal excretion of MTX than healthy subjects, which might lead to adverse effects of MTX. Furthermore, we hypothesized that oral indican might also alter the pharmacokinetics of various acidic pharmaceuticals which were substrates of OATs and/or MRPs beyond MTX [37,38], such as penicillines, cephalosporins, fluoroquinolones, nonsteroidal anti-inflammatory drugs, HMG-CoA reductase inhibitors and antiviral drugs. Therefore, we suggested that for the sake of safety, concurrent use of indican or indican-containing herbs such as I. indigotica, P. tinctorium and P. perfoliatum with acidic drugs should be with caution especially for CKD patients. In conclusion, oral indican significantly increased the systemic exposure and MRT of MTX through inhibition on multiple anion transporters including OAT 1, OAT 3 and MRP 4.
Acknowledgement
The work was, in part, supported by the Ministry of Science and Technology, Taiwan (MOST 105-2314-B-039-018 and MOST 106-2320-B-039-009) and China Medical University Hospital, Taichung, Taiwan (DMR-105-006, DMR-106-138 and DMR-106-139).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jfda.2017.11.006.
Funding Statement
The work was, in part, supported by the Ministry of Science and Technology, Taiwan (MOST 105-2314-B-039-018 and MOST 106-2320-B-039-009) and China Medical University Hospital, Taichung, Taiwan (DMR-105-006, DMR-106-138 and DMR-106-139).
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Gilbert KG, Maule HG, Rudolph B, Lewis M, Vandenburg H, Sales E, et al. Quantitative analysis of indigo and indigo precursors in leaves of Isatis spp. and Polygonum tinctorium. Biotechnol Prog. 2004;20:1289–92. doi: 10.1021/bp0300624. [DOI] [PubMed] [Google Scholar]
- 2. Zhang QG, Wei F, Liu Q, Chen LJ, Liu YY, Luo F, et al. The flavonoid from Polygonum perfoliatum L. inhibits herpes simplex virus 1 infection. Acta Virol. 2014;58:368–73. doi: 10.4149/av_2014_04_368. [DOI] [PubMed] [Google Scholar]
- 3. Ho YL, Chang YS. Studies on the antinociceptive, anti-inflammatory and anti pyretic effects of Isatis indigotica root. Phytomedicine. 2002;9:419–24. doi: 10.1078/09447110260571661. [DOI] [PubMed] [Google Scholar]
- 4. Hsuan SL, Chang SC, Wang SY, Liao TL, Jong TT, Chien MS, et al. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol. 2009;123:61–7. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. da Silva IB, Rangel IL, de Leite Lima RM, Lima EO, de Medeiros PL, Leite SP. Antibacterial and antifungal activities of indican (indoxyl-D-glucoside) Afr J Pharm Pharmacol. 2016;10:200–5. [Google Scholar]
- 6. Jang HG, Heo BG, Park YS, Namiesnik J, Barasch D, Katrich E, et al. Chemical composition, antioxidant and anticancer effects of the seeds and leaves of indigo (Polygonum tinctorium Ait.) plant. Appl Biochem Biotechnol. 2012;167(7):1986–2004. doi: 10.1007/s12010-012-9723-7. [DOI] [PubMed] [Google Scholar]
- 7. Hou YC, Tsai SY, Chan SL, Yang SY, Chao PD. Indoxyl sulfate, a uremic toxin, is biotransformed from indoxyl-beta-D-glucoside (indican) in rats. Toxicon. 2008;52:440–4. doi: 10.1016/j.toxicon.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 8. Yu CP, Sweet DH, Peng YH, Hsieh YW, Chao PL, Hou YC, et al. Effects of nonsteroidal anti-inflammatory drugs on the renal excretion of indoxyl sulfate, a nephro-cardiovascular toxin, in rats. Eur J Pharmaceut Sci. 2017;101:66–70. doi: 10.1016/j.ejps.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 9. Kaminski TW, Pawlak K, Karbowska M, Mysliwiec M, Pawlak D. Indoxyl sulfate - the uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017;18:35. doi: 10.1186/s12882-017-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23:1892–901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- 11. Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niwa T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120. Ther Apher Dial. 2011;15:120–4. doi: 10.1111/j.1744-9987.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 13. Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13:1711–20. doi: 10.1097/01.asn.0000022017.96399.b2. [DOI] [PubMed] [Google Scholar]
- 14. Masereeuw R, Mutsaers HA, Toyohara T, Abe T, Jhawar S, Sweet DH, et al. The kidney and uremic toxin removal: glomerulus or tubule? Semin Nephrol. 2014;34:191–208. doi: 10.1016/j.semnephrol.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 15. Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, et al. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLos One. 2011;6:e18438. doi: 10.1371/journal.pone.0018438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang YK, Wang YJ, Gupta P, Chen ZS. Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J. 2015;17:802–12. doi: 10.1208/s12248-015-9757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uwai Y, Taniguchi R, Motohashi H, Saito H, Okuda M, Inui K. Methotrexate-loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metabol Pharmacokinet. 2004;19:369–74. doi: 10.2133/dmpk.19.369. [DOI] [PubMed] [Google Scholar]
- 19. Vlaming ML, van Esch A, van de Steeg E, Pala Z, Wagenaar E, van Tellingen O, et al. Impact of abcc2 [multidrug resistance-associated protein (MRP) 2], abcc3 (MRP3), and abcg2 (breast cancer resistance protein) on the oral pharmacokinetics of methotrexate and its main metabolite 7-hydroxy-methotrexate. Drug Metabol Dispos. 2011;39:1338–44. doi: 10.1124/dmd.111.038794. [DOI] [PubMed] [Google Scholar]
- 20. Chioukh R, Noel-Hudson MS, Ribes S, Fournier N, Becquemont L, Verstuyft C. Proton pump inhibitors inhibit methotrexate transport by renal basolateral organic anion transporter hOAT3. Drug Metabol Dispos. 2014;42:2041–8. doi: 10.1124/dmd.114.058529. [DOI] [PubMed] [Google Scholar]
- 21. Keysser G. Methotrexate toxicity. Myths and facts. Z Rheumatol. 2011;70:108–13. doi: 10.1007/s00393-010-0687-0. [DOI] [PubMed] [Google Scholar]
- 22. Montaudie H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol. 2011;25(Suppl 2):12–8. doi: 10.1111/j.1468-3083.2011.03991.x. [DOI] [PubMed] [Google Scholar]
- 23. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncol. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 24. Oberthür C, Graf H, Hamburger M. The content of indigo precursors in Isatis tinctoria leaves–a comparative study of selected accessions and post-harvest treatments. Phytochemistry. 2004;65:3261–8. doi: 10.1016/j.phytochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 25. Horhota ST, Fung HL. Validity of oral bioavailability estimates of phenolsulfonphthalein based on total urinary excretion from rats. J Pharm Sci. 1978;67:267–8. doi: 10.1002/jps.2600670240. [DOI] [PubMed] [Google Scholar]
- 26. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27. Yu CP, Hsieh YC, Shia CS, Hsu PW, Chen JY, Hou YC, et al. Increased systemic exposure of methotrexate by a polyphenol-rich herb via modulation on efflux transporters multidrug resistance-associated protein 2 and breast cancer resistance protein. J Pharm Sci. 2016;105:343–9. doi: 10.1016/j.xphs.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 28. Jansen J, Fedecostante M, Wilmer MJ, Peters JG, Kreuser UM, van den Broek PH, et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep. 2016;6:26715. doi: 10.1038/srep26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int. 2004;65:162–74. doi: 10.1111/j.1523-1755.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 30. Gault MH, Koch B, Dossetor JB. Phenolsulfonphthalein (PSP) in assessment of renal function. J Am Med Assoc. 1967;200:871–3. [PubMed] [Google Scholar]
- 31. Itagaki S, Sugawara M, Kobayashi M, Nishimura S, Fujimoto M, Miyazaki K, et al. Major role of organic anion transporters in the uptake of phenolsulfonphthalein in the kidney. Eur J Pharmacol. 2003;475:85–92. doi: 10.1016/s0014-2999(03)02111-3. [DOI] [PubMed] [Google Scholar]
- 32. Nomura M, Motohashi H, Sekine H, Katsura T, Inui K. Developmental expression of renal organic anion transporters in rat kidney and its effect on renal secretion of phenolsulfonphthalein. Am J Physiol Ren Physiol. 2012;302:F1640–9. doi: 10.1152/ajprenal.00525.2011. [DOI] [PubMed] [Google Scholar]
- 33. Smeets PH, van Aubel RA, Wouterse AC, van den Heuvel JJ, Russel FG. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol. 2004;15:2828–35. doi: 10.1097/01.ASN.0000143473.64430.AC. [DOI] [PubMed] [Google Scholar]
- 34. El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Therapeut. 2007;320:229–35. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- 35. Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Therapeut. 2002;302:666–71. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 36. Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, et al. European uremic toxin work group. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–70. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 38. Burckhardt G. Drug transport by organic anion transporters (OATs) Pharmacol Ther. 2012;136:106–30. doi: 10.1016/j.pharmthera.2012.07.010. [DOI] [PubMed] [Google Scholar]