Summary
Targeted restoration of immunological tolerance to self-antigens or innocuous environmental allergens represents the ultimate aim of treatment options in autoimmune and allergic disease. Antigen-specific immunotherapy (ASI) is the only intervention that has proven disease-modifying efficacy as evidenced by induction of long-term remission in a number of allergic conditions. Mounting evidence is now indicating that specific targeting of pathogenic T cells in autoinflammatory and autoimmune settings enables effective restoration of immune homeostasis between effector and regulatory cells and alters the immunological course of disease. Here, we discuss the key lessons learned during the development of antigen-specific immunotherapies and how these can be applied to inform future interventions. Armed with this knowledge and current high-throughput technology to track immune cell phenotype and function, it may no longer be a matter of ‘if’ but ‘when’ this ultimate aim of targeted tolerance restoration is realised.
Keywords: immunotherapy, immune tolerance, allergy, autoimmunity, immunoregulation
Introduction
The treatment of allergy and autoimmunity urgently requires novel therapeutic approaches; current medical interventions broadly aim to manage symptoms of disease but do not address their underlying cause, i.e. loss of immunological tolerance. Immunosuppressive drugs have both short- and long-term adverse effects, most importantly compromised immune function in immune surveillance of cancer and protection from infectious diseases. A major benefit to antigen-specific immunotherapy (ASI) is that it has the potential to modify disease with reduced reliance on conventional broad-range systemic immunosuppression.
Allergy is an incredibly common health concern, affecting more than 20% of the population in developed countries [1], with prevalence in the UK being one of the highest reported globally (estimated 44% of adults) [2]. Despite prevalence of allergic diseases reaching an epidemic scale, clinical focus has remained on maintaining an allergen-free lifestyle and access to anti-histamines and epinephrine rather than specific treatments.
The prevalence of autoimmune conditions has also risen steadily in recent decades, with current estimates suggesting one in eight people worldwide have at least one autoimmune condition [3]. Autoimmune diseases often require lifelong therapy with immunosuppressive drugs which at best slow down disease progression, therefore, new specific treatments represent a major advance in the field. We believe that the goal of novel approaches should be to target disease-associated antigens and suppress allergen-specific or autoreactive T cells that recognise them in order to re-instate immunological balance.
Antigen-specific immunotherapy: a historical perspective
‘Immunological tolerance’ was formally defined in Peter Medawar’s Nobel Prize winning speech as a ‘state of indifference or non-reactivity towards a substance that would normally be expected to excite an immunological response’ [4]. Prior to this definition, research investigating manipulation of immunity to generate a state of non-reactivity was underway. Specific tolerance induction was documented in the scientific literature as early as 1827. Dakin described the indigenous practice of ingesting poison ivy leaves to reduce poison ivy rash [5], i.e. tolerance induction via delivery of the offending antigen.
Pioneers in the field first published clinical applications of specific tolerance in 1911, with Wells and Osborne utilising the mucosal route of delivery in guinea pigs, inducing systemic non-responsiveness by feeding vegetable proteins [6], and Noon and Freeman using increasing subcutaneous doses of grass pollen extract to desensitise a hay-fever sufferer [7]. At the time, allergic reactions were assumed to be caused by antigenic ‘toxins’. Injection of small doses of antigen (‘toxin’) was therefore predicted to induce ‘anti-toxins’ to neutralise the threat. Although we now appreciate allergens are not toxins, their early observations that delivery of whole allergen could re-establish non-reactivity to these antigens was correct. Interestingly, Noon also noted a transient reduction in resistance after high doses of allergen prior to resistance increasing to above its prior level, indicative of transient immune response before establishing robust immune regulation. Induction of antigen-specific T cell anergy preceded by short-term T cell activation has been shown to be a feature of both allergen and autoantigen tolerance induction [8–10].
In the >100 years since these early reports, there has been steady interest in allergen immunotherapy (AIT) and significant clinical data supporting its disease moderating impact [11,12]. Improvements in antigen production, standardisation, and purity have significantly improved safety and efficacy such that subcutaneous and/or sublingual allergen delivery have shown efficacy in prevention of bee venom [13, 14], house dust mite [10], grass pollen [15–17], peanut [18, 19], milk [20, 21], cat dander [22], and birch pollen allergies [23, 24]. At present, however, ASI is yet to be fully translated into autoimmune disease treatment regimes.
Developing ASI for autoimmune diseases: lessons from the field of allergen immunotherapy
Parallel development of antigen-specific immunotherapy interventions for autoimmune and allergic diseases has facilitated considerable knowledge transfer between the disciplines. In both settings, over-active antigen-specific T and B cells can be controlled by administration of antigen or antigenic peptides. Importantly, approaches used in the clinic today for allergy are safe to administer, do not exacerbate disease flares and are able to establish potent immune regulation to alter disease course.
Target antigen
The correct antigen(s) must be targeted to achieve disease suppression. In allergy, this can be more straightforward; identifiable symptoms are usually triggered by single or a small number of antigens; however, complexity can arise if patients are sensitised to a broad range of allergens. Purified protein antigen reduces the risk of potentially immunogenic contaminants in crude extracts, including innate pattern-recognition receptor ligands.
Recombinant allergen proteins represent the gold-standard for immunotherapeutic applications, allowing for tightly controlled purity of antigen to be produced in high quantity. Recombinant grass [25] and Bet v 1 (birch) allergens [26] have been tested in patients with similar safety and efficacy to natural antigen. Genetically modified recombinant antigens have been designed with mutated IgE-binding motifs or as fragmented constitutive overlapping peptides to reduce the risk of IgE cross-linking, while maintaining T cell reactivity and represent a powerful tool for engineering a safer product for desensitisation [27–29].
The complexity of autoimmune diseases poses a significant challenge to antigen identification. Immune responses vary considerably between patients and at different time points of disease progression [30, 31]. At present, our knowledge of disease-initiating and propagating autoantigens in many autoimmune diseases is incomplete and further complicated by epitope spreading [32]. Despite this, ASI has shown promise in inducing tolerance towards specific auto-antigens. A series of studies in the 1980–90’s indicated that disease in rodent models of autoimmune disease including experimental autoimmune encephalomyelitits (EAE) [33, 34], collagen-induced arthritis [35], and non-obese-diabetes [36] could be ameliorated by ASI. More recently, clinical trials utilising tolerogenic peptides in the treatment of multiple sclerosis (MS), type 1 diabetes, systemic lupus erythematosus, and Graves’ disease have been safe, well tolerated and indicate that disease severity can be lessened [37–39]. Such trials are the outcome of decades of research into the identification of relevant auto-antigens and T cell epitopes in these diseases.
Experience has shown that when the pathogenic autoantigen is defined, e.g. thyroid-stimulating hormone receptor (TSHR) in Graves’ disease, it is possible to target disease pathogenesis and deliver clinical benefit. Where the autoantigen(s) responsible are not fully defined or disease is driven by reactivity to multiple antigens, it is possible to control disease severity by targeting only one antigen within the same affected tissue via bystander or linked suppression.
Immune regulation: the need for active suppression and bystander regulation
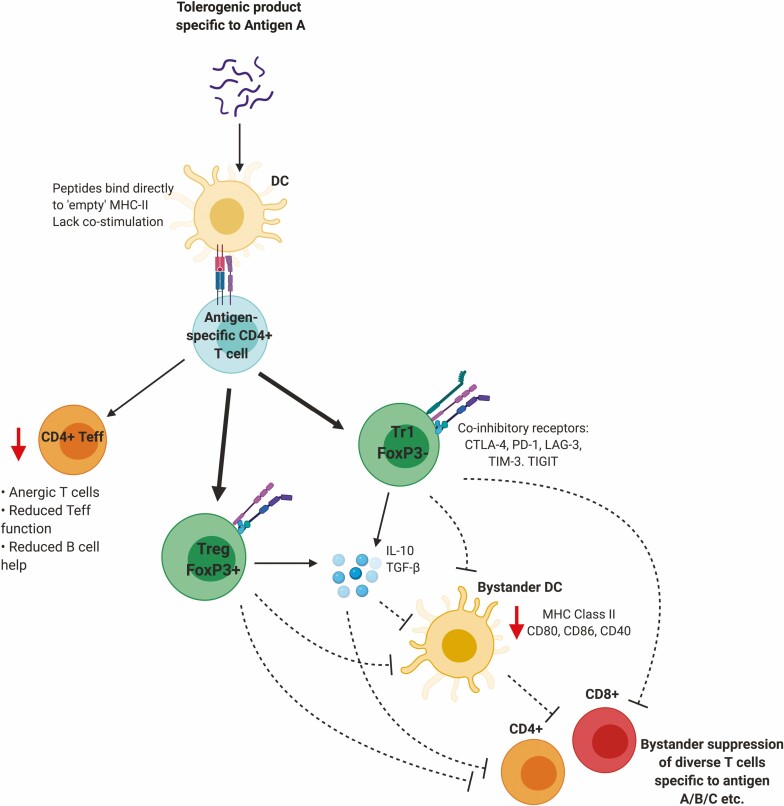
Linked suppression occurs when antigen-specific T cell tolerance induction to an immunodominant epitope of antigen A leads to suppression of immune responses against other epitopes within antigen A. Bystander suppression enables antigen-specific T cells directed against antigen A to indirectly dampen immune responses against antigens B, C, and so on, by involvement of T cell-mediated suppression of antigen presenting cells and neighbouring T cells (Fig. 1). Both linked and bystander suppression have been reported outcomes of ASI in multiple allergic and autoimmune disease settings.
Figure 1.
Proposed mechanism of action of bystander suppression. Antigen-specific immunotherapies prevent the generation and activation of CD4+ Teff and instead divert Tconv CD4+ cells towards anergy and also promote the expansion of antigen-specific Tr1-like cells and/or Treg. Both tolerised Tr1-like and Treg can exert cell-contact mediated and cytokine mediated suppression (dashed lines) on APC and non-antigen-specific T cells to ultimately prevent T cell activation in a non-antigen-specific manner.
The processes by which this localised antigen-independent suppression occurs are still poorly understood, although bystander suppression plays an identifiable role in murine peptide tolerance models of EAE and in allergic contexts [40, 41]. In cat allergy, tolerance induction using 12 Fel d1 peptides not only suppressed patient responses to these Fel d1 peptides, but also to Fel d1 peptides not included in the therapy [42].
IL-10, secreted by anergic Type 1 regulatory-like (Tr1-like) cells, regulatory T cells (Treg), regulatory B cells (Breg), and tolerogenic dendritic cells, is thought to be central in establishing broader regulation following antigen-specific therapy [43]. Its role in establishing bystander suppression is likely due to its ability to downregulate costimulatory molecules and MHC-II on the surface of antigen-presenting cells (APC) [44–46], thus reducing antigen-presentation and T cell priming potency of APC. IL-10 is also able to directly suppress both T and B cell responses via inhibition of co-stimulatory signalling [47–49]. This not only suppresses subsequent immune responses to the initial antigen targeted, but also other disease-relevant antigens nearby in the inflamed tissue.
Tolerance-induced Tr1-like and Treg express high levels of coinhibitory receptors CTLA-4, LAG-3, PD-1, TIM-3, and TIGIT [50, 51]. The inhibitory receptors control T cell signalling through mechanisms including competition with ligands/counter receptors, engagement of protein phosphatases and inhibitory signalling. Collectively, they act as checkpoints and fine tune the magnitude of the T cell response to antigen [52].
TGF-β is highly expressed by Treg as a result of oral antigen delivery [53] and contributes to prevention of EAE when disease is initiated via myelin basic protein (MBP) or proteolipid protein – indicating strong bystander control of multiple antigen specificities in complex disease [54]. Targeting antigens to the liver induces Treg in a TGF-β-dependent manner [55] and has also been shown to generate multi-antigen tolerance induction [56].
Antigen-specific immunotherapies based on single antigen specificities are unlikely to be effective in complex and dynamic multi-antigen diseases such as type 1 diabetes and rheumatoid arthritis, unless they can evoke bystander suppression [57]. Therefore, understanding the mechanism of bystander suppression and how best to incorporate it into antigen-specific immunotherapy will prove crucial to resolve the dilemma of which antigen(s) to target in a specific disease. Providing that tolerance induction towards a dominant antigen is sufficient to control the pathogenic nature of T cells of multiple antigen specificities, disease severity should be ameliorated.
Mechanism of action and associated risks
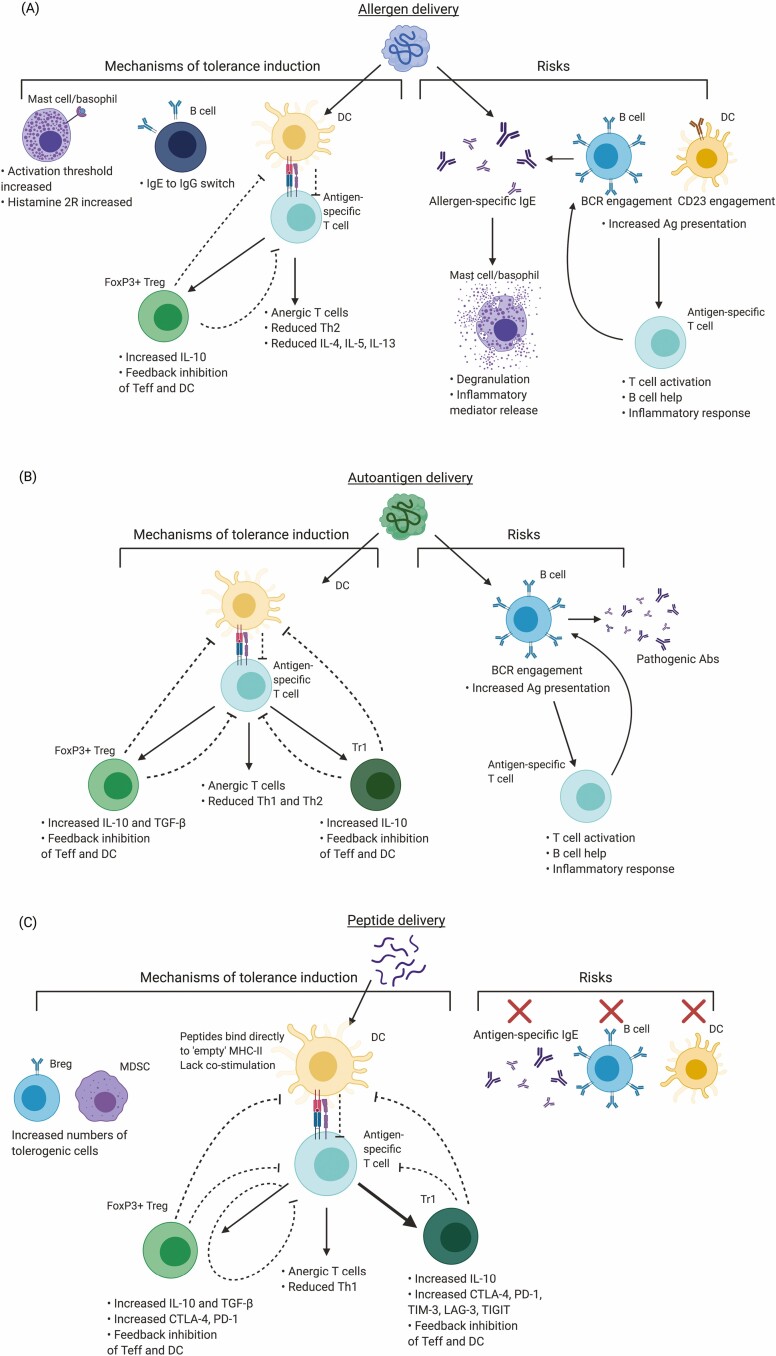
Through careful investigation of ASI/AIT using either intact allergen, autoantigen, or antigenic peptides, we now have a good understanding of the cellular and molecular mechanisms involved in tolerogenic antigen delivery and the risks associated with each type of approach (summarised in Fig. 2).
Figure 2.
Summarised mechanisms of action of ASI/AIT and associated risks. Antigen-specific immunotherapies have varying mechanisms of action and potential risks depending on whether they utilise (A) intact allergen, (B) intact autoantigen, or (C) peptides representing T cell epitopes of either allergen or autoantigen. Promotion of activity denoted by black arrows, inhibition of activity denoted by black dashed lines and mitigation of risks denoted by red crosses.
Allergen immunotherapy using intact allergen commonly results in a decrease of allergen-specific effector T cell (Teff) number and/or functionality, often described as a Th2→Th1 population shift, although we would argue this is often related to a change in ratio between these populations as opposed to Th2 converting to Th1 [8, 58–60]. Regulatory populations are elevated after treatment; some studies report a dominant FoxP3+ Treg effect while others report primarily FoxP3-like [8, 61]. This may be disease-specific, related to the nature of antigen delivered, treatment protocol used, or simply the design of immunological readouts. The consensus, however, is that peripherally induced regulatory T cells are expanded after treatment and contribute to disease control. AIT also moderates basophil and mast cell degranulation, increasing the threshold required for their activation, in addition to increasing expression of histamine receptor 2 to act as a histamine ‘sink’ [17, 62]. IL-10+ are promoted by AIT intervention [63–65]. Most importantly, allergen-sequestering IgG4 titres are increased. IgG4 competes directly with IgE for antigen-binding sites, reducing the likelihood of early phase immune response on subsequent exposure [13, 65–67]. Of the 4 IgG subclasses, IgG4 has the lowest abundance, accounting for around 4% of serum IgG, yet it can reach up to 75% IgG after AIT [68]. IgG4 has several ‘anti-inflammatory’ properties, due to its low affinity for Fcγ receptors, inability to activate complement and ability to form bivalent antibodies which are not able to cross-link antigen to form immune complexes [69, 70]. The production of IgG4 is mediated by plasmablasts/plasma cells [71, 72] differentiated from IL-10+, where IL-10 promotes the generation of ‘blocking’ IgG4 antibodies, while inhibiting IL-4-mediated IgE class-switching in humans [17, 73, 74]. Although the IgG4-mediated suppression of IgE is well documented, more recently, evidence has emerged suggesting that antibodies of different classes, particularly IgG2, can also play a role in blocking IgE engagement [72, 75, 76]. Decrease in allergen-specific IgE has been observed after long-term treatment duration (1–2 years), occurring much later than symptomatic relief [17].
Conversely, there are well-documented risks associated with use of whole allergen: even very low doses of pure antigen can cause unpredictable cross-linking of IgE and activation of mast cells and basophils via the high-affinity IgE receptor FcεR1. IgE-antigen complex bound by the lower affinity IgE receptor, CD23, on B cells and DC promotes antigen uptake and efficient presentation to T cells [77, 78], perpetuating allergen-specific IgE production, T cell priming, and activation. Furthermore, conformational epitopes of antigen can directly bind B cell receptors (BCR) for BCR cross-linking [79].
ASI directed towards autoimmune diseases also initially used whole autoantigen as the tolerising agent. Early trials in MS injected intact MBP isolated from human, porcine, or bovine sources and did not promote immunological or symptomatic improvement [80–82]. The delivery of whole antigen proved to be high risk, due to the potential generation of pathogenic antibodies [83]. As such, considerable progress was made to properly identify relevant T cell epitopes in murine models and MS patients [57, 84–86] for use in peptide immunotherapy (PIT).
Peptides representing T cell epitopes have also been employed in the allergy field, as peptides avoid IgE-mediated immune responses and unpredictable immunological effects associated with the use of whole allergen [87]. Short soluble peptide epitopes are unable to cross link IgE and are unlikely to provide the 3D-conformation required to function as B cell epitopes. Peptides are significantly less likely to result in mast cell and basophil degranulation compared to whole allergen [22, 88]. The mechanisms of tolerance induction when utilising whole allergen versus peptide-based approaches, are likely to be subtly different, although direct mechanistic comparison studies between the two parallel approaches are lacking at present. Akdis and colleagues showed that peptide immunotherapy did not generate B cell tolerance – one of the key features reported via use of whole allergen in AIT. However, these experiments did generate ‘blocking’ IgG4 antibodies and a relative reduction in IgE [13, 14].
Where whole antigen requires processing by APC for presentation to T cells, peptides representing disease-relevant T cell epitopes specifically utilise resting DC in lymphoid organs for presentation to cognate T cells without the need for antigen processing [89]. Steady-state DC (ssDC) are tolerogenic and well-suited to promote the restoration of Teff versus Treg balance. A proportion of MHC Class II on ssDCs are ‘empty’ or transiently loaded with low-affinity peptides [90]; therefore, exogenous peptides delivered can bind directly to MHC-II for presentation to CD4+ T cells. ssDC provide low levels of costimulation (CD80/CD86) to T cells and are less efficient in antigen uptake and presentation [91, 92]. As such, antigen-specific T cells do not receive sufficient stimulatory signal from T cell receptor (TCR) engagement alone to become activated [93] and are instead diverted into a state of functional anergy [94] by repeated antigen exposure in which they no longer respond to antigen via classical inflammatory signalling pathways but instead exert a regulatory phenotype. Antigen-specific naive and effector CD4+ T cells become regulatory Tr1-like cells (FoxP3-) and FoxP3+ Treg throughout PIT [95] and express high levels of IL-10 and co-inhibitory receptors (CTLA-4, PD-1, TIGIT, LAG-3) [51]. As a result, T cell immunity directed towards the antigen is quenched; readouts often include significant reduction in Teff cytokine production (IFN-γ, IL-2 in autoimmunity; IL-4, IL-5 in allergy) [96].
Peptide design must reflect naturally processed T cell epitopes, with high solubility and minimal aggregate potential. Studies in MS using an altered peptide ligand warned the field that using non-native peptides could result in disease exacerbation [97, 98]. These adverse effects primarily arose due to administration of an excessively high dose of peptide which may not have remained soluble in vivo, hence promoting rather than suppressing immunity. This story highlights the need for peptides used in antigen-targeting immunotherapies to be highly soluble and to mimic the naturally processed T cell epitope to avoid unforeseen immunological consequences. These risks were avoided in later clinical trials utilising natural T cell epitope peptides with high solubility [37, 99].
Route of administration
Tolerance induction via mucosal surfaces (oral, nasal, sublingual) has been popular historically, as these sites are exposed continually to environmental antigens and yet in healthy individuals do not generate immune responses to these stimuli.
Seminal experiments pioneered by Weiner and colleagues in a number of animal autoimmune diseases models, showed overwhelming efficacy of fed antigen to prevent disease [53]. Oral tolerance was notably less effective in pre-sensitised animals (which better reflect ongoing disease in humans) [100]. Unfortunately, in clinical trials, oral tolerance induction in MS using MBP was deemed to be safe but ineffective [101]. This is most likely due to the relative low doses of antigen used in patients compared to those tested in animals [102] and to generally ‘weak’ immune responses towards autoantigens.
Even in allergic diseases where the antigen typically generates stronger immune responses, oral delivery of antigen does not consistently achieve tolerance. An exception to this is peanut allergy, in which repeated doses of pure peanut protein increasing up to 800 mg were shown to decrease peanut sensitivity after 30 weeks of treatment. Patients were not followed up after treatment had ended, therefore the longevity of reduced sensitivity and the requirement for maintenance therapy was not assessed [103]. Delivery of the offending antigen to the site of hypersensitivity may co-opt natural regulatory feedback loops in situ for disease modification. Such a significant amount of protein would be extremely expensive when requiring recombinant allergens, and highly inefficient due to degradation within the stomach prior to having any tolerogenic effect in the gut.
Mucosal delivery via sublingual immunotherapy (SLIT) and systemic delivery via subcutaneous immunotherapy (SCIT) routes offer clinical efficacy using much lower doses of antigen and are now common practice in allergen immunotherapy [11, 12]. Few studies compare the efficacy of SCIT versus SLIT directly, making an over-arching judgement on the validity of each method difficult; however, the mechanism of action is likely to be subtly different [104, 105].
Intralymphatic antigen delivery is early in development, but has shown remarkable efficacy in murine models [106] and in clinical trials of allergy [107, 108]. Direct delivery of grass pollen allergen intralymphatically has generated safe, pain-free, and effective allergen-specific tolerance much more rapidly than standard SCIT therapy (8 weeks with 3 injections vs. 3 years therapy with 54 injections). Allergy symptoms and allergen-specific IgE were significantly reduced after both treatment courses and maintained for 2 years post-treatment. It is likely that this approach is transferable across allergies, upcoming trials will be followed with interest.
In the context of autoimmune disease, thorough pre-clinical investigation in mouse models of disease have shown a hierarchy of delivery route efficacy, with subcutaneous > intranasal > oral delivery [109]. As such, clinical trials in relapsing remitting MS and Graves’ disease were performed by subcutaneous/intradermal delivery of tolerogenic peptides. No unexpected safety concerns arose during these trials, and both displayed significant decreases in disease severity by the end of treatment course [37, 110]. Importantly, studies in experimental animal models have shown that s.c. injection of soluble peptides are detected on the surface of ssDC within minutes [89]. Naive T cell encounter with the epitope presenting ssDC transiently signal via their TCR, as evidenced by ERK phosphorylation followed by transient IL-2 secretion; however, both ERK phosphorylation and inflammatory cytokine secretion are reduced with further antigen administration. Repeated delivery of soluble peptide leads to induction of IL-10 expression in the anergic T cells [109, 40].
The application of ASI via the intralymphatic route (DIAGNODE trial) in autoimmune disease used direct injection of glutamic acid decarboxylase antigen into lymph nodes of type 1 diabetes patients, with a promising reduction in insulin requirement after treatment [112, 113]. This alteration in delivery route may be a more potent means of generating immune tolerance, as suggested by murine and allergy studies; however, this approach is less practical for tolerance maintenance.
Dosing strategy and longevity of response
Dose escalation has been a cornerstone of allergen immunotherapy ever since Freeman and Noon’s very first clinical intervention in hayfever [7]; however, little mechanistic data has been collected to validate exactly how dose-escalation benefits tolerance induction in allergy. Dosage is scaled up from initially minute amounts, which avoids induction of severe immune reactions, while enabling a higher maintenance dose to be achieved [11]. A higher acceptable maximum dosage is linked to improved immunological outcomes with increased IL-10 production and antibody switch towards IgG4 [17].
Mechanistically speaking, more has been learned about dose escalation from the perspective of peptide ASI in autoimmunity. Burton and colleagues performed detailed immunophenotyping during successful dose-escalation immunotherapy using MBPAc1-9 [4Y] and showed that antigen-specific T cells undergo a progressive alteration in T cell transcriptional programme rendering them resistant to production of inflammatory cytokines. Dose escalation is fundamental to reach high peptide doses, which could generate adverse effects if delivered singularly, and these higher doses are vital to the generation of suppressive IL-10-producing Tr1-like cells which express high levels of coinhibitory receptors [51]. Recent work has identified that antigen-specific T cells in this system undergo epigenetic priming as a result of dose escalation to inhibit inflammatory transcription factors and effector cytokines [111]. It is highly likely that similar processes are occurring in allergen-specific T cells during dose escalation, but this specific data is yet to be collected.
Based on current evidence, it appears that antigen-specific tolerance induction and consequent disease-modifying benefit will persist alongside continued exposure to tolerising antigen. In a study of beekeepers naturally exposed to venom, T cell regulation and a switch to IL-10 secreting Tr1-like cells was established and maintained during exposure to antigen during the bee season, after which reactivity returned to baseline 2–3 months later [8]. In cat allergy and grass pollen desensitisation, a reduction in allergic symptoms was reported to persist 2–3 years post-treatment cessation [22, 114, 115]. Particularly with airborne allergens, it may be almost impossible to avoid continued natural exposure to intact allergen, and this may play a supporting role in mediating long-term T and B cell tolerance skewed towards IgG4 for maintenance of allergen-specific tolerance.
Peptide immunotherapy trials in multiple sclerosis and Graves’ disease suppressed disease flares during treatment course, although in both cases patients did not enter a permanent state of immunological tolerance. Suppression of immune pathology was observed for around 1 month after the end of treatment, which reflects tolerance duration induced in euthymic mice [116]. However, it is worth noting that these treatment periods were relatively short, each running for 16–18 weeks of peptide dosing. There may be a longer lasting benefit with longer treatment.
As such, to maintain immunological tolerance and disease control, it is likely that ASI would need to be maintained over a significant period of time, particularly in the case of autoimmune diseases. For patients to undergo repeated antigen exposure on a regular basis without significantly impacting quality of life, a delivery system in which patients can self-administer treatment would be highly beneficial. This may involve tablet formulations for gut delivery or microneedle patches already used for intradermal insulin delivery [117]. Any successful therapeutic approach must avoid induction of anti-drug antibodies or non-specific immune suppression [118].
Direct tolerogenic peptide delivery and novel carrier-based approaches
While it is clear that peptides representing CD4+ T cell epitopes can promote peripheral tolerance and hence suppress autoimmune diseases, various additional approaches have been described. We know that ssDC both induce and maintain peripheral tolerance [92]. Monocyte-derived DC (moDC) can be generated in vitro from peripheral blood monocytes and have tolerogenic properties when cultured in the presence of NFκB inhibitors [119] or vitamin D3 [120]. moDC generated from patients with RA have been incubated with disease-associated peptides and injected back into the patient showing that this approach is safe with evidence of immune modulation [121, 122]. Nanoparticles (NP) have been designed to be taken up by DC, monocytes or liver sinusoidal endothelial cells (LSEC). Various approaches to targeting the immunosuppressive environment in the liver have been taken. We know that ageing red blood cells are recycled via hepatocytes in the liver [123]. Kontos and colleagues developed approaches for targeting antigens to red blood cells in vivo [124]. In a further development of this technology, Anokion are now testing direct modification of antigens by glycosylation to target liver receptors. Furthermore, Lutterotti and colleagues are building on their previous work with antigenic peptides coupled to mononuclear cells [125] by coupling peptides to red blood cells with ethylene carbodiimide. Carambia and colleagues have described the design of ferromagnetic nanoparticles coupled with antigen. These selectively target LSECs and induce systemic tolerance in mice in a TGF-β-dependent fashion [55, 126].
NPs are taken up by different APC depending on their size. Small NP are endocytosed by DC; Kishimoto and colleagues have delivered rapamycin to DC with antigen in order to induce regulatory T cells [127]. Larger NP containing antigen is phagocytosed by macrophages in order to create a suppressive immune response [128]. Preclinical work describing encapsulation of gliadin [129] has led to a clinical trial of gliadin NP in coeliac disease. Santamaria and colleagues have described a sophisticated NP delivery approach. Here NP are coupled to MHC class II molecules and incubated with peptide epitopes. These MHC-II-NP do not activate naive T cells but promote IL-10 production by antigen-specific Th1 cells [130]. The induction of Tr1-like cells by MHC-II-NP was recently shown to mediate bystander suppression of autoimmune responses in the liver [56, 131].
How best to deliver antigens for tolerance induction
Is it necessary to couple antigens to NP for tolerance induction? The use of NP arose from early studies in which it was shown that peptide epitopes can induce an allergic response in vivo [132]. In our experience, however, the balance between a peptide epitope being tolerogenic rather than immunogenic is determined by its solubility. Furthermore, peptides themselves directly target tolerogenic DC in vivo when designed to mimic naturally processed antigens. Our original observations showed that some but not all T cell epitopes induce tolerance when administered in a soluble form [133]. Peptides must be designed to bind MHC II in a conformation that mimics the naturally processed epitope in order to induce tolerance. This is consistent with our recent observation that tolerogenic peptides bind directly to steady state DC in vivo. DCs collected from lymphoid tissues following subcutaneous injection of soluble peptide are able to induce tolerance following adoptive transfer in mice [89]. Furthermore, insoluble peptides fail to reach lymphoid DC following subcutaneous injection and are immunogenic rather than tolerogenic. However, these peptides are rendered tolerogenic by increasing their solubility. The first rule governing design of peptides for tolerance induction is, therefore, peptides must mimic naturally processed epitopes when bound to their MHC restriction element.
Peptides must be soluble such that they rapidly distribute throughout the body and bind to MHC II on ssDC in lymphoid organs.
Peptides should induce cytokines that promote bystander suppression such that an epitope from antigen A within a tissue can suppress the response of antigens B, C, and D from the same tissue. This is a critical feature of antigen-specific immunotherapy in those diseases where there are a range of antigens, i.e. multiple sclerosis, rheumatoid arthritis, and type I diabetes.
Peptides with the properties listed above are defined as antigen processing independent epitopes or apitopes.
ASI using tolerogenic peptides: mechanism of action and translation to the clinic
Our recent work has defined the detailed mechanism of how tolerogenic peptides function in vivo. Our original work compared mucosal routes of administration. Oral delivery of peptides was ineffective due to proteolytic destruction [116] whereas nasal administration induced bystander suppression in a dose dependent fashion [9, 44, 134]. Peptide therapy induced cells with a Tr1-like, IL-10 secreting phenotype [135] that mediated suppression by downregulating the antigen presenting properties of DCs [136]. The mechanism by which soluble, tolerogenic peptides convert potentially pathogenic T cells into Tr1-like cells was revealed in recent studies. First, Burton et al. showed that repeated encounter with peptides presented by ssDC induced antigen-specific CD4 T cell anergy and suppressed secretion of inflammatory cytokines [51]. Analysis of gene expression in cells showed that peptide treatment caused a marked upregulation in expression of genes encoding inhibitory receptors PD1, CTLA4, Lag3, Tim3, and TIGIT and transcription factors known to promote expression of IL-10 such as c-Maf. This transcriptional signature was also been seen in other Tr1-like cells and in tumour infiltrating lymphocytes [137]. Later, our work has revealed the link between antigen-exposure, T cell signalling, and the subsequent expression of IL-10 and the generation of Tr1-like cells. The anergy seen among T cells in peptide-induced tolerance results from a membrane proximal block in cell signalling causing a loss of inflammatory cytokine gene expression [95]. Bevington et al. have shown that this reduced level of cell signalling is insufficient to drive the epigenetic changes required for transcription of inflammatory genes; however, epigenetic priming of genes associated with tolerance renders them sensitive to reduced levels of transcription factors [111]. This novel mechanism explains how cells including tumour infiltrating lymphocytes and cells rendered tolerant with either peptide antigens or anti-CD3 antibodies [138] change their transcriptional landscape with selective upregulation of genes encoding inhibitory receptors, transcription factors such as c-Maf and the anti-inflammatory cytokine IL-10. Furthermore, the detailed understanding of how tolerogenic peptides modulate the immune response to antigen provides the foundation for their application in treatment of hypersensitivity diseases including autoimmune and allergic diseases.
Antigen-specific immunotherapy with apitopes has been tested in four clinical trials in two autoimmune diseases with distinct immune pathologies. Multiple sclerosis is a cell-mediated disease with various disease-associated antigens. Two phase 1 followed by a phase 2 clinical trials have shown that treatment with a cocktail of four HLA-DR2 binding peptides from MBPAc1-9 was sufficient to significantly suppress inflammation in the CNS as measured by gadolinium enhanced MRI [37, 99] and to improve cognition in patients with relapsing MS. In Graves’ disease autoimmunity is caused by antibodies specific for TSHR. Two dominant HLA-DR3 binding peptides suppressed immune responses in HLA-DR transgenic mice [139]. Furthermore, intradermal injection of these peptides normalised thyroid hormone secretion in 7/10 patients with mild-to-moderate hyperthyroidism in a phase 1 trial. Most importantly, the results of these four clinical trials shows that treatment with soluble peptides designed as apitopes is well tolerated with promising signs of efficacy. It is important to add that these clinical trials used a dose-escalation protocol shown to promote Tr1-like cell generation in pre-clinical models. Recent studies with peptide immunotherapy in coeliac disease have proved the importance of dose escalation [140]. The dose-escalation protocol shown to induce Tr1-like cells through epigenetic modification of the genome in experimental animal models [111] has proved to be the preferred approach for effective tolerance induction in the clinic. Further analysis of antigen-specific T cells in future clinical trials of antigen-specific immunotherapy is required to confirm that this is due to selective epigenetic priming at tolerance-associated genes.
Concluding statement
Antigen-specific immunotherapy remains the ‘holy-grail’ for selective treatment of allergies and autoimmune diseases. Rapid advances in our understanding of the mechanisms involved provide options ranging from the administration of tolerogenic DC, through design of sophisticated NP to simple delivery of apitopes. Critical issues including mechanism of action, bystander suppression, ease of manufacture, and successful translation to the clinic will determine success of each approach for treatment of hypersensitivity diseases.
Acknowledgements
The Editor-in-Chief, Tim Elliott, and handling editor, Menno van Zelm, would like to thank the following reviewer, Willem van de Veen, and an anonymous reviewer, for their contribution to the publication of this article. Figures created with BioRender.com.
Glossary
Abbreviations
- AIT
Allergen immunotherapy
- APC
Antigen-presenting cells
- ASI
Antigen-specific immunotherapy
- BCR
B cell receptors
- Breg
Regulatory B cells
- EAE
Experimental autoimmune encephalomyelitits
- LSEC
Liver sinusoidal endothelial cells
- MBP
Myelin basic protein
- MHC-II-NP
MHC class II conjugated nanoparticles
- moDC
Monocyte-derived DC
- MS
Multiple sclerosis
- NP
Nanoparticles
- PIT
Peptide immunotherapy
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- ssDC
Steady-state DC
- TCR
T cell receptor
- Teff
Effector T cell
- Tr1-like
Type 1 regulatory-like
- Treg
Regulatory T cells
- TSHR
Thyroid-stimulating hormone receptor.
Contributor Information
Naomi Richardson, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK.
David Cameron Wraith, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK.
Funding
This work was supported by the University of Birmingham and research grant from the Children’s Liver Disease Foundation (NR).
Author contributions
Authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. Authors are accountable for all aspects of accuracy and integrity of the work.
Conflict of interest
D.C.W. is Professor of Immunology at the University of Birmingham and CSO and Founder of Apitope International NV. N.R. declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability
The data underlying this article are cited in the reference list and available in the public domain.
References
- 1. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J 2014;7:12. 10.1186/1939-4551-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy ML, Price D, Zheng X et al. Inadequacies in UK primary care allergy services: national survey of current provisions and perceptions of need. Clin Exp Allergy 2004;34:518–9. 10.1111/j.1365-2222.2004.1945.x [DOI] [PubMed] [Google Scholar]
- 3. Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis 2015;3:151–5. 10.12691/IJCD-3-4-8 [DOI] [Google Scholar]
- 4. Medawar R. Nobel lecture. NobelPrize.org. Nobel Media AB 2021. Available at: https://www.nobelprize.org/prizes/medicine/1960/medawar/lecture/ [Google Scholar]
- 5. Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci 1829;4:98–100. [Google Scholar]
- 6. Wells H, Osborne T. The biological reactions of the vegetable proteins. I. Anaphylaxis. J Infect Dis 1911;8:66–124. 10.1093/infdis/8..66 [DOI] [Google Scholar]
- 7. Noon L. Prophylactic inoculation against hay fever. Lancet 1911;177:1572–3. 10.1159/000228032 [DOI] [Google Scholar]
- 8. Meiler F, Zumkehr J, Klunker S et al. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. 2008;205:2887–98. 10.1084/jem.20080193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabrysová L, Wraith DC. Antigenic strength controls the generation of antigen-specific IL-10-secreting T regulatory cells. Eur J Immunol 2010;40:1386–95. 10.1002/eji.200940151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoyne GF, Askonas BA, Hetzel C et al. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Int Immunol 1996;8:335–42. 10.1093/intimm/8.3.335 [DOI] [PubMed] [Google Scholar]
- 11. Jutel M, Agache I, Bonini S et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015;136:556–68. 10.1016/j.jaci.2015.04.047 [DOI] [PubMed] [Google Scholar]
- 12. Jutel M, Agache I, Bonini S et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol 2016;137:358–68. 10.1016/j.jaci.2015.12.1300 [DOI] [PubMed] [Google Scholar]
- 13. Akdis CA, Akdis M, Blesken T et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest 1996;98:1676–83. 10.1245/s10434-010-1366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller U, Akdis CA, Fricker M et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol 1998;101:747–54. 10.1016/S0091-6749(98)70402-6 [DOI] [PubMed] [Google Scholar]
- 15. Ebner C, Siemann U, Bohle B et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy 1997;27:1007–15. 10.1111/j.1365-2222.1997.tb01252.x [DOI] [PubMed] [Google Scholar]
- 16. Durham SR, Yang WH, Pedersen MR et al. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006;117:802–9. 10.1016/j.jaci.2005.12.1358 [DOI] [PubMed] [Google Scholar]
- 17. Francis JN, James LK, Paraskevopoulos G et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008;121:1120–5.e2. 10.1016/j.jaci.2008.01.072 [DOI] [PubMed] [Google Scholar]
- 18. Jones SM, Pons L, Roberts JL et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol 2009;124:292–300. 10.1016/j.jaci.2009.05.022.Clinical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wasserman RL, Hague AR, Pence DM et al. Real-world experience with peanut oral immunotherapy: lessons learned from 270 patients. J Allergy Clin Immunol Pract 2019;7:418–26. 10.1016/j.jaip.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 20. Skripak JM, Nash SD, Rowley H et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2008;122:1154–60. 10.1016/j.jaci.2008.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keet CA, Seopaul S, Knorr S et al. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2013;132:737–9.e6. 10.1016/j.jaci.2013.05.006.Long-Term [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oldfield WLG, Larché M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: A randomised controlled trial. Lancet 2002;360:47–53. 10.1016/S0140-6736(02)09332-7 [DOI] [PubMed] [Google Scholar]
- 23. Möbs C, Slotosch C, Löffler H et al. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol 2010;184:2194–203. 10.4049/jimmunol.0901379 [DOI] [PubMed] [Google Scholar]
- 24. Pfaar O, Bachert C, Kuna P et al. Sublingual allergen immunotherapy with a liquid birch pollen product in patients with seasonal allergic rhinoconjunctivitis with or without asthma. J Allergy Clin Immunol 2019;143:970–7. 10.1016/j.jaci.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 25. Jutel M, Jaeger L, Suck R et al. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol 2005;116:608–13. 10.1016/j.jaci.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 26. Pauli G, Larsen TH, Rak S et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol 2008;122:951–60. 10.1016/j.jaci.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 27. Pellaton C, Perrin Y, Boudousquié C et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy 2013;3:17. 10.1186/2045-7022-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klimek L, Bachert C, Lukat KF et al. Allergy immunotherapy with a hypoallergenic recombinant birch pollen allergen rBet v 1-FV in a randomized controlled trial. Clin Transl Allergy 2015;5:28. 10.1186/s13601-015-0071-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campana R, Marth K, Zieglmayer P et al. Vaccination of nonallergic individuals with recombinant hypoallergenic fragments of birch pollen allergen Bet v 1: Safety, effects, and mechanisms. J Allergy Clin Immunol 2019;143:1258–61. 10.1016/j.jaci.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazza G, Ponsford M, Lowrey P et al. Diversity and dynamics of the T-cell response to MBP in DR2+ve individuals. Clin Exp Immunol 2002;128:538–47. 10.1046/j.1365-2249.2002.01831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ponsford M, Mazza G, Coad J et al. Differential responses of CD45+ve T-cell subsets to MBP in multiple sclerosis. Clin Exp Immunol 2001;124:315–22. https://doi:10.1046/j.1365-2249.2001.01507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol 1996;8:831–6. 10.1016/B978-044451271-0.50003-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol 1988;140:440–5. Available from: https://www.jimmunol.org/content/140/2/440 [PubMed] [Google Scholar]
- 34. Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol 1988;112:364–70. 10.1016/0165-5728(92)90258-M [DOI] [PubMed] [Google Scholar]
- 35. Thompson HS, Staines NA. Gastric administration of type II collagen delays the onset and severity of collagen-induced arthritis in rats. Clin Exp Immunol 1986;64:581–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1542438/ [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang JZ, Davidson L, Eisenbarth G et al. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. J Endocrinol Invest 1994;17:573–80. 10.1007/BF03347752 [DOI] [PubMed] [Google Scholar]
- 37. Chataway J, Martin K, Barrell K et al. ; ATX-MS1467 Study Group. Effects of ATX-MS-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology 2018;90:e955–62. 10.1212/WNL.0000000000005118 [DOI] [PubMed] [Google Scholar]
- 38. Zimmer R, Scherbarth HR, Rillo OL et al. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis 2013;72:1830–5. 10.1136/annrheumdis-2012-202460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alhadj Ali M, Liu YF, Arif S et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med 2017;9:402,eaaf7779. 10.1126/scitranslmed.aaf7779 [DOI] [PubMed] [Google Scholar]
- 40. Bevington SL, Ng STH, Britton GJ et al. Chromatin priming renders T cell tolerance-associated genes sensitive to activation below the signaling threshold for immune response genes. Cell Rep 2020;31:107748. 10.1016/j.celrep.2020.107748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med 1991;174:791–8. 10.1084/jem.174.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol 1998;28:1251–61. [DOI] [PubMed] [Google Scholar]
- 43. Campbell JD, Buckland KF, McMillan SJ et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med 2009;206:1535–47. 10.1084/jem.20082901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ng TH, Britton GJ, Hill EV et al. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol 2013;4:129. 10.3389/fimmu.2013.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sundstedt A, O’Neill EJ, Nicolson KS et al. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol 2003;170:1240–8. 10.4049/jimmunol.170.3.1240 [DOI] [PubMed] [Google Scholar]
- 46. Perona-Wright G, Anderton SM, Howie SE et al. IL-10 permits transient activation of dendritic cells to tolerize T cells and protect from central nervous system autoimmune disease. Int Immunol 2007;19:1123–34. 10.1093/intimm/dxm084 [DOI] [PubMed] [Google Scholar]
- 47. Corinti S, Albanesi C, la Sala A et al. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol 2001;166:4312–8. 10.4049/jimmunol.166.7.4312 [DOI] [PubMed] [Google Scholar]
- 48. Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol 1995;154:4341–50. Available from: https://www.jimmunol.org/content/154/9/4341 [PubMed] [Google Scholar]
- 49. Taylor A, Akdis M, Joss A et al. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol 2007;120:76–83. 10.1016/j.jaci.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 50. Smith LK, Boukhaled GM, Condotta SA et al. Interleukin-10 directly inhibits CD8+ T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity 2018;48:299–312.e5. 10.1016/j.immuni.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. White AM, Wraith DC. Tr1-like T cells - an enigmatic regulatory T cell lineage. Front Immunol 2016;5:1–13. 10.3389/fimmu.2016.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burton BR, Britton GJ, Fang H et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun 2014;5:1–13. 10.1038/ncomms5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thaventhiran T. T cell co-inhibitory receptors-functions and signalling mechanisms. J Clin Cell Immunol 2012;S12 ,1–12. 10.4172/2155-9899.s12-004 [DOI] [Google Scholar]
- 54. Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol 1999;73:153–264. 10.1016/S0065-2776(08)60787-7 [DOI] [PubMed] [Google Scholar]
- 55. Chen Y, Kuchroo VK, Inobe J et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 1994;265:1237–40. 10.1126/science.7520605 [DOI] [PubMed] [Google Scholar]
- 56. Carambia A, Freund B, Schwinge D et al. TGF-β-dependent induction of CD4+CD25+Foxp3 + Tregs by liver sinusoidal endothelial cells. J Hepatol 2014;61:594–9. 10.1016/j.jhep.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 57. Umeshappa CS, Singha S, Blanco J et al. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat Commun 2019;10:1–17. 10.1038/s41467-019-09893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol 1998;28:1251–61. [DOI] [PubMed] [Google Scholar]
- 59. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol 2006;6:761–71. 10.1038/nri1934 [DOI] [PubMed] [Google Scholar]
- 60. Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 2015;8:17. 10.1186/s40413-015-0063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Möbs C, Ipsen H, Mayer L et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol 2012;130:1108–16.e6. 10.1016/j.jaci.2012.07.056 [DOI] [PubMed] [Google Scholar]
- 62. Kniemeyer O, Brakhage AA, Ferreira F et al. Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 2016;167:1067–78.e16. 10.1016/j.cell.2016.09.050 [DOI] [PubMed] [Google Scholar]
- 63. Novak N, Mete N, Bussmann C et al. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J Allergy Clin Immunol 2012;130:1153–8.e2. 10.1016/j.jaci.2012.04.039 [DOI] [PubMed] [Google Scholar]
- 64. Van De Veen W, Stanic B, Yaman G et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 2013;131:1204–12. 10.1016/j.jaci.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 65. Boonpiyathad T, Meyer N, Moniuszko M et al. High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers. Allergy 2017;72:407–15. 10.1111/all.12966 [DOI] [PubMed] [Google Scholar]
- 66. Zissler UM, Jakwerth CA, Guerth FM et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. Ebiomedicine 2018;36:475–88. 10.1016/j.ebiom.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spertini F, DellaCorte G, Kettner A et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: Results of a phase IIb study. J Allergy Clin Immunol 2016;138:162–8. 10.1016/j.jaci.2016.02.044 [DOI] [PubMed] [Google Scholar]
- 68. Shamji MH, Ljørring C, Francis JN et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012;67:217–26. 10.1111/j.1398-9995.2011.02745.x [DOI] [PubMed] [Google Scholar]
- 69. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol 2014;133:621–31. 10.1016/j.jaci.2013.12.1088 [DOI] [PubMed] [Google Scholar]
- 70. van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol 1986;64:415–22. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1542347/pdf/clinexpimmunol00122-0193.pdf [PMC free article] [PubMed] [Google Scholar]
- 71. van der Neut Kolfschoten M, Schuurman J, Losen M et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007;317:1554–7. 10.1126/science.1144603 [DOI] [PubMed] [Google Scholar]
- 72. Mattoo H, Mahajan VS, Della-Torre E et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134:679–87. 10.1016/j.jaci.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heeringa JJ, Karim AF, van Laar JAM et al. Expansion of blood IgG4+ B, TH2, and regulatory T cells in patients with IgG4-related disease. J Allergy Clin Immunol 2018;141:1831–1843.e10. 10.1016/j.jaci.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 74. Oo YH, Weston CJ, Lalor PF et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol 2010;184:2886–98. 10.4049/jimmunol.0901216 [DOI] [PubMed] [Google Scholar]
- 75. Satoguina JS, Weyand E, Larbi J et al. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol 2005;174:4718–26. 10.4049/jimmunol.174.8.4718 [DOI] [PubMed] [Google Scholar]
- 76. Dodev TS, Bowen H, Shamji MH et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy 2015;70:720–4. 10.1111/all.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sánchez Acosta G, Kinaciyan T, Kitzmüller C et al. IgE-blocking antibodies following SLIT with recombinant Mal d 1 accord with improved apple allergy. J Allergy Clin Immunol 2020;146:894–900.e2. 10.1016/j.jaci.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 78. Kehry MR, Yamashita LC. Low-affinity IgE receptor (CD23) function on mouse B cells: role in IgE-dependent antigen focusing. Proc Natl Acad Sci USA 1989;86:7556–60. 10.1073/pnas.86.19.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Novak N, Allam JP, Hagemann T et al. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol 2004;114:364–70. 10.1016/j.jaci.2004.05.038 [DOI] [PubMed] [Google Scholar]
- 80. Pali-Schöll I, Jensen-Jarolim E. The concept of allergen-associated molecular patterns (AAMP). Curr Opin Immunol 2016;42:113–8. 10.1016/j.coi.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 81. Campbell B, Vogel PJ, Fisher E et al. Myelin basic protein administration in multiple sclerosis. Arch Neurol 1973;29:10–5. 10.1001/archneur.1973.00490250028003 [DOI] [PubMed] [Google Scholar]
- 82. Gonsette RE, Delmotte P, Demonty L. Failure of basic protein therapy for multiple sclerosis. J Neurol 1977;216:27–31. 10.1007/BF00312812 [DOI] [PubMed] [Google Scholar]
- 83. Romine JS, Salk J, Wiederholt WC et al. Studies on myelin basic protein administration in multiple sclerosis patients. In: Bauer HJ, Poser S, Ritter GE (eds), Progress in Multiple Sclerosis Research. Berlin, Heidelberg: Springer Berlin Heidelberg, 1980:419–27. [Google Scholar]
- 84. Genain CP, Abel K, Belmar N et al. Late complications of immune deviation therapy in a nonhuman primate. Science 1996;274:2054–7. 10.1126/science.274.5295.2054 [DOI] [PubMed] [Google Scholar]
- 85. Wraith DC, Smilek DE, Mitchell DJ et al. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell 1989;59:247–55. 10.1016/0092-8674(89)90287-0 [DOI] [PubMed] [Google Scholar]
- 86. Fukaura H, Kent SC, Pietrusewicz MJ et al. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest 1996;98:70–7. 10.1172/JCI118779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tuohy VK, Yu M, Yin L et al. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev 1998;164:93–100. 10.1111/j.1600-065X.1998.tb01211.x [DOI] [PubMed] [Google Scholar]
- 88. Larché M. Peptide therapy for allergic diseases: Basic mechanisms and new clinical approaches. Pharmacol Ther 2005;108:353–61. 10.1016/j.pharmthera.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 89. Oldfield WL, Kay AB, Larché M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol 2001;167:1734–9. 10.4049/jimmunol.167.3.1734 [DOI] [PubMed] [Google Scholar]
- 90. Shepard ER, Wegner A, Hill EV et al. The mechanism of action of antigen processing independent T cell epitopes designed for immunotherapy of autoimmune diseases. Front Immunol 2021;12:654201. 10.3389/fimmu.2021.654201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Santambrogio L, Sato AK, Carven GJ et al. Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci USA 1999;96:15056–61. 10.1073/pnas.96.26.15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hawiger D, Inaba K, Dorsett Y et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001;194:769–79. 10.1084/jem.194.6.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685–711. 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- 94. Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol 1989;7:445–80. 10.1146/annurev.iy.07.040189.002305 [DOI] [PubMed] [Google Scholar]
- 95. Gimmi CD, Freeman GJ, Gribben JG et al. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA 1993;90:6586–90. 10.1073/pnas.90.14.6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Anderson PO, Manzo BA, Sundstedt A et al. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur J Immunol 2006;36:1374–85. 10.1002/eji.200635883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gardner LM, O’Hehir RE, Rolland JM. High dose allergen stimulation of T cells from house dust mite-allergic subjects induces expansion of IFN-gamma+ T Cells, apoptosis of CD4+IL-4+ T cells and T cell anergy. Int Arch Allergy Immunol 2004;133:1–13. 10.1159/000075248 [DOI] [PubMed] [Google Scholar]
- 98. Bielekova B, Goodwin B, Richert N et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000;6:1167–75. 10.1038/80516 [DOI] [PubMed] [Google Scholar]
- 99. Kappos L, Comi G, Panitch H et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 2000;6:1176–82. 10.1038/80525 [DOI] [PubMed] [Google Scholar]
- 100. Streeter HB, Rigden R, Martin KF et al. Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurol Neuroimmunol Neuroinflamm 2015;2:e93. 10.1212/NXI.0000000000000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Conde AA, Stransky B, Faria AM et al. Interruption of recently induced immune responses by oral administration of antigen. Braz J Med Biol Res 1998;31:377–80. 10.1590/S0100-879X1998000300008 [DOI] [PubMed] [Google Scholar]
- 102. Benson JM, Stuckman SS, Cox KL et al. Oral administration of myelin basic protein is superior to myelin in suppressing established relapsing experimental autoimmune encephalomyelitis. J Immunol 1999;162:6247–54. Available from: https://www.jimmunol.org/content/162/10/624 [PubMed] [Google Scholar]
- 103. Anagnostou K, Islam S, King Y et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 2014;383:1297–304. 10.1016/S0140-6736(13)62301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schulten V, Tripple V, Aasbjerg K et al. Distinct modulation of allergic T cell responses by subcutaneous vs. sublingual allergen-specific immunotherapy. Clin Exp Allergy 2016;46:439–48. 10.1586/14737175.2015.1028369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lawrence MG, Steinke JW, Borish L. Basic science for the clinician: mechanisms of sublingual and subcutaneous immunotherapy. Ann Allergy Asthma Immunol 2016;117:138–42. 10.1016/j.anai.2016.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Martínez-Gómez JM, Johansen P, Erdmann I et al. Intralymphatic injections as a new administration route for allergen-specific immunotherapy. Int Arch Allergy Immunol 2009;150:59–65. 10.1159/000210381 [DOI] [PubMed] [Google Scholar]
- 107. Senti G, Prinz Vavricka BM, Erdmann I et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci USA 2008;105:17908–12. 10.1073/pnas.0803725105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Senti G, Crameri R, Kuster D et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol 2012;129:1290–6. 10.1016/j.jaci.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 109. Burton BR, Britton GJ, Fang H et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun 2014;5:4741. 10.1038/ncomms5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pearce SHS, Dayan C, Wraith DC et al. Antigen-specific immunotherapy with thyrotropin receptor peptides in graves’ hyperthyroidism: a phase I study. Thyroid 2019;29:1003–11. 10.1089/thy.2019.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bevington SL, Ng STH, Britton GJ, Keane P, Wraith DC, Cockerill PN. Chromatin Priming Renders T Cell Tolerance-Associated Genes Sensitive to Activation below the Signaling Threshold for Immune Response Genes. Cell Rep 2020. 31:107748. 10.1016/j.celrep.2020.107748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ludvigsson J, Faresjö M, Hjorth M et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–20. 10.1056/NEJMoa0804328 [DOI] [PubMed] [Google Scholar]
- 113. Ludvigsson J, Wahlberg J, Casas R. Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 2017;376:697–9. 10.1056/nejmc1616343 [DOI] [PubMed] [Google Scholar]
- 114. Durham SR, Emminger W, Kapp A et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol 2012;29:717–25. 10.1016/j.jaci.2011.12.973 [DOI] [PubMed] [Google Scholar]
- 115. Couroux P, Patel D, Armstrong K et al. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy 2015;45:974–81. 10.1111/cea.12488 [DOI] [PubMed] [Google Scholar]
- 116. Metzler B, Wraith DC. Inhibition of T-cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T-cell-dependent functions. Immunology 1999;97:257–63. 10.1046/j.1365-2567.1999.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hultström M, Roxhed N, Nordquist L. Intradermal insulin delivery: a promising future for diabetes management. J Diabetes Sci Technol 2014;8:453–7. 10.1177/1932296814530060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Krishna M, Nadler SG. Immunogenicity to biotherapeutics - the role of anti-drug immune complexes. Front Immunol 2016;7:1–13. 10.3389/fimmu.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Martin E, O’Sullivan B, Low P et al. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity 2003;18:155–67. 10.1016/S1074-7613(02)00503-4 [DOI] [PubMed] [Google Scholar]
- 120. Piemonti L, Monti P, Sironi M et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 2000;164:4443–51. 10.4049/jimmunol.164.9.4443 [DOI] [PubMed] [Google Scholar]
- 121. Benham H, Nel HJ, Law SC et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med 2015;7:290ra87. 10.1126/scitranslmed.aaa9301 [DOI] [PubMed] [Google Scholar]
- 122. Bell GM, Anderson AE, Diboll J et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis 2017;76:227–34. 10.1136/annrheumdis-2015-208456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grewal PK. The Ashwell-Morell receptor. Methods Enzymol 2010;479:223–41. 10.1016/S0076-6879(10)79013-3 [DOI] [PubMed] [Google Scholar]
- 124. Kontos S, Kourtis IC, Dane KY et al. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci USA 2013;110:E60–8. 10.1073/pnas.1216353110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lutterotti A, Yousef S, Sputtek A et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med 2013;5:188ra75. 10.1126/scitranslmed.3006168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Carambia A, Freund B, Schwinge D et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol 2015;62:1349–56. 10.1016/j.jhep.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 127. LaMothe RA, Kolte PN, Vo T et al. Tolerogenic nanoparticles induce antigen-specific regulatory T cells and provide therapeutic efficacy and transferrable tolerance against experimental autoimmune encephalomyelitis. Front Immunol 2018;9:1–11. 10.3389/fimmu.2018.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Getts DR, Shea LD, Miller SD et al. Harnessing nanoparticles for immune modulation. Trends Immunol 2015;36:419–27. 10.1016/j.it.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Freitag TL, Podojil JR, Pearson RM et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology 2020;158:1667–81. 10.1053/j.gastro.2020.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Clemente-Casares X, Blanco J, Ambalavanan P et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016;530:434–40. 10.1038/nature16962 [DOI] [PubMed] [Google Scholar]
- 131. Umeshappa CS, Mbongue J, Singha S et al. Ubiquitous antigen-specific T regulatory type 1 cells variably suppress hepatic and extrahepatic autoimmunity. J Clin Invest 2020;130:1823–9. 10.1172/JCI130670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pedotti R, Mitchell D, Wedemeyer J et al. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol 2001;2:216–22. 10.1038/85266 [DOI] [PubMed] [Google Scholar]
- 133. Anderton SM, Viner NJ, Matharu P et al. Influence of a dominant cryptic epitope on autoimmune T cell tolerance. Nat Immunol 2002;3:175–81. 10.1038/ni756 [DOI] [PubMed] [Google Scholar]
- 134. Anderton SM, Manickasingham SP, Burkhart C et al. Fine specificity of the myelin-reactive T cell repertoire: implications for TCR antagonism in autoimmunity. J Immunol 1998;161:3357–64. Available at: https://www.jimmunol.org/content/161/7/3357 [PubMed] [Google Scholar]
- 135. Burkhart C, Liu GY, Anderton SM et al. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol 1999;11: 1625–34. 10.1093/intimm/11.10.1625 [DOI] [PubMed] [Google Scholar]
- 136. Gabrysová L, Nicolson KS, Streeter HB et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med 2009;206:1755–67. 10.1084/jem20082118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chihara N, Madi A, Kondo T et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018;558:454–9. 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mayo L, Cunha AP, Madi A et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 2016;139:1939–57. 10.1093/brain/aww113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jansson L, Vrolix K, Jahraus A et al. Immunotherapy with apitopes blocks the immune response to TSH receptor in HLA-DR transgenic mice. Endocrinology 2018;159:3446–57. 10.1210/en.2018-00306 [DOI] [PubMed] [Google Scholar]
- 140. Truitt KE, Daveson AJM, Ee HC et al. Randomised clinical trial: a placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment Pharmacol Ther 2019;50:547–55. 10.1111/apt.15435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are cited in the reference list and available in the public domain.