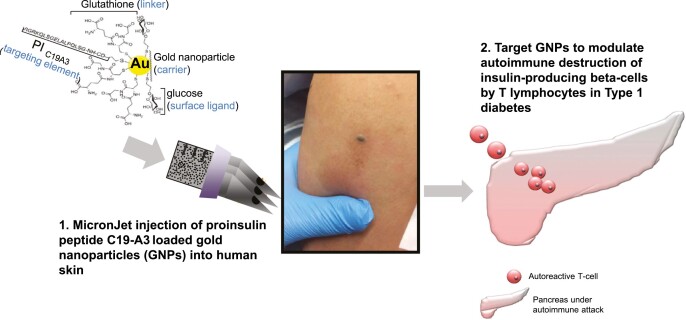
Abstract
Antigen-specific immunotherapy is an immunomodulatory strategy for autoimmune diseases, such as type 1 diabetes, in which patients are treated with autoantigens to promote immune tolerance, stop autoimmune β-cell destruction and prevent permanent dependence on exogenous insulin. In this study, human proinsulin peptide C19-A3 (known for its positive safety profile) was conjugated to ultrasmall gold nanoparticles (GNPs), an attractive drug delivery platform due to the potential anti-inflammatory properties of gold. We hypothesised that microneedle intradermal delivery of C19-A3 GNP may improve peptide pharmacokinetics and induce tolerogenic immunomodulation and proceeded to evaluate its safety and feasibility in a first-in-human trial. Allowing for the limitation of the small number of participants, intradermal administration of C19-A3 GNP appears safe and well tolerated in participants with type 1 diabetes. The associated prolonged skin retention of C19-A3 GNP after intradermal administration offers a number of possibilities to enhance its tolerogenic potential, which should be explored in future studies
Keywords: gold nanoparticle, peptide immunotherapy, microneedle, type 1 diabetes, proinsulin
Graphical Abstract
Introduction
Autoimmune destruction of insulin-producing β-cell by T lymphocytes in type 1 diabetes (T1D) makes affected individuals permanently dependent on exogenous insulin. Despite advances in insulin delivery with glucose sensors and closed-loop systems, in many regions of the world less than 30% of patients achieve glycaemic control sufficient to prevent long-term complications [1]. Immunotherapy aims to stop β-cell destruction, thus preserving the individual’s ability to synthesise endogenous insulin. This could lead to better control, or even disease prevention [2, 3]. Antigen-specific immunotherapy (ASI) is an attractive immunomodulatory strategy for antigen-specific autoimmune diseases, such as T1D in which patients are treated with autoantigens to promote immune tolerance. The aim is to boost immunoregulation, by expanding antigen-specific regulatory T-cells, although some ASI appears to act predominantly by deleting or anergising antigen-specific effector cells [4–8]. The advantage of this approach is that it can potentially slow the disease process in a targeted manner, without the need for systemic immunosuppression. Human studies have been conducted in this area [8, 9] and a range of approaches have been used including intramuscular injection of whole antigen alone or with adjuvant [10–14], administration of the antigen via the mucosal route (orally or nasally) [15–18], DNA vaccination [19, 20], and the use of peptide epitopes or altered peptide ligands [21]. These approaches have generally proved safe and very well tolerated but achieving sufficient efficacy to reliably reverse established disease in humans has not yet been demonstrated [8, 14, 16, 17, 22]. A review of the antigen-specific immunotherapy trials to date suggests that the context in which the antigen is presented to the immune system plays an important role in efficacy [23–26].
In this study, the HLA-DR4 (DRB1∗0401) restricted proinsulin peptide C19-A3 has been selected as the therapeutic immunomodulatory antigen as it is associated with a positive safety profile in Phase 1 clinical studies and is thought to preserve β-cell function by modulating autoreactive CD4 T-cells [27, 28]. In addition, this peptide has been conjugated to gold nanoparticles (GNPs), an attractive drug delivery platform in this context due to the potential anti-inflammatory properties of gold [29], to create a C19-A3 GNP construct. Previous in vitro studies conducted in our laboratories administered the C19-A3 GNP construct into the skin using hollow microneedles and exemplified the diffusive properties of the C19-A3 GNP construct, which facilitated its reflux from the point of dermal deposition to the overlying human skin epidermis. These studies demonstrated maintained stability of the construct upon delivery and efficient uptake by antigen-presenting cells in the skin, with the responsible antigen-presenting cells being identified as predominantly Langerhans cells [30], which are considered to have tolerogenic properties by some researchers [31, 32], whilst others favour the tolerogenic potential of other skin dendritic cells [33, 34]. Importantly, the use of GNP provides the possibility of introducing a second cargo to accompany the peptide to further enhance the tolerogenic properties of the construct. Thus, the microneedle-administered C19-A3 GNP construct may offer a valuable platform for tolerogenic immunomodulation in T1D and, therefore, the aim of this study was to evaluate the safety of the construct in a first-in-human clinical trial.
Materials and methods
Study design
The study was a two-centre, open-label, uncontrolled, single-group first-in-human Phase 1A safety study of C19-A3 GNP peptide in individuals with T1D.
The Investigational medicinal product (IMP) was C19-A3 GNP (Midacore™), which comprises GNPs [30, 35] of a size of less than 5 nm, covalently coupled to an 18-amino acid human peptide, the sequence of which is identical to the residues from position 19 in the C-peptide of proinsulin through to position 3 on the A-chain of the same molecule (GSLQPLALEGSLQKRGIV). The peptide is synthesised with a linker to facilitate binding to the GNPs: 3-mercaptopropionyl-SLQPLALEGSLQKRGIV 2 acetate salt (disulfide bond). The chemical composition of the IMP contained a ratio of 4 C19-A3 peptides: 11 glucose C2: 29 glutathione ligands as determined by 1H-NMR (proton nuclear magnetic resonance). The nanoparticle suspension was purified using Amicon ultra centrifugal filtration units with a 10 kDa cut-off and sterile pre-filtered through a poly(ether sulfone) (PES) 0.22 µm pre-sterilised cartridge compliant for use in GMP manufacturing. Liquid chromatography–mass spectrometry (LC–MS) analysis showed that peptide integrity was maintained in the nanoparticles. Stability testing was also performed both on the nanoparticle product stored at 4oC for 23 weeks and at 25oC for 10 weeks. The IMP was manufactured at Midatech Pharma Plc (Derio, Spain) and delivered as a sterilised solution in small vials produced by Baccinex SA (Courroux, Switzerland). Following the filling process of the IMP stability batch (No. F15220), the structure of the peptide was checked both by 1H-NMR and by LC–MS for the release of the drug product. Both methods confirmed the integrity of C19-A3 peptide. The IMP was stored, QP released, and shipped to the clinical sites by PharmaKorell GmbH (Lörrach, Germany). A typical batch contained: [C19-A3 peptide] = 1.33 mg/ml; [gold] = 5.5 mg/ml; [glucose linker] = 0.6 mg/ml; [glutathione linker] = 1.79 mg/ml. As the drug substance was diluted 1:7 to 1:10, depending on the content of C19-A3 peptide per particle, and as 50 μl of the diluted solution was administered to the study participants, this corresponded to C19-A3 peptide: 10 μg; gold: 39 μg; glucose linker: 4.3 μg; glutathione: 12.7 μg. The vials were stored refrigerated at 2–8°C.
C19-A3 GNP was administered intradermally in the deltoid region of the arm via CE-marked 600 µm length MicronJet600™ hollow microneedles (NanoPass Technologies Ltd.) attached to a standard luer-lock syringe. The device has been used in over 50 clinical trials, mostly for the delivery of vaccines [36–40], but also for multiple early-phase trials as well as Phase III study in allergy immunotherapy for the purpose of inducing tolerance (https://clinicaltrials.gov/).
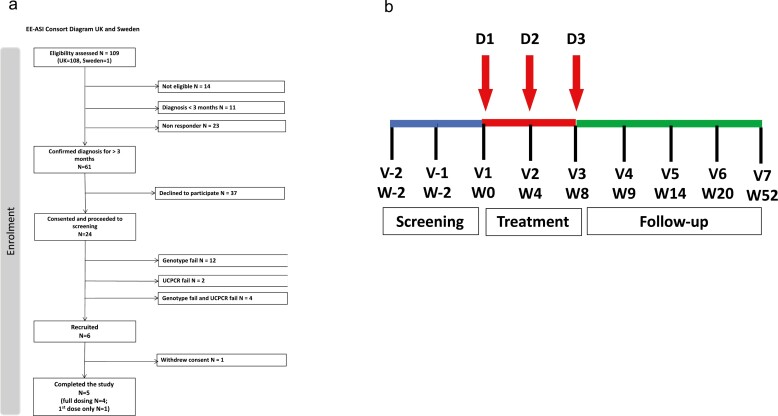
Three doses were given at 4-weekly intervals (weeks 0, 4, and 8) in alternate arms (2 doses in one arm and 1 dose in the other arm). The single-dose given in 50 μl volume was equivalent to 10 μg of C19-A3 peptide (cumulative dose of 30 μg). Participants were followed up according to the full study protocol for up to 52 weeks in total (Fig. 1).
Figure 1.
Study recruitment and visit schedule. (a) Consort diagram and (b) schedule of the study visits. D, dose; V, visit; W, week.
Inclusion criteria were as follows: age (16–40 years of age), >3 months from a clinical diagnosis of T1D (dated from the day of first insulin injection), commenced on insulin within 1 month of diagnosis, HLA-DRB1∗0401 genotype and 2-h post-meal urine C-peptide to creatinine ratio (UCPCR) >0.2 nmol/mmol or random serum C-peptide >0.06 nmol/l on at least one occasion (maximum three tests on different days). The main exclusion criteria were the use of immunosuppressive or immunomodulatory therapies, immunisation with live or killed vaccinations or allergic desensitisation procedures less than 1 month before the first treatment, raised serum creatinine or abnormal urine albumin/creatinine ratio (ACR), HbA1c >86 mmol/mol, recent participation in other research trials of immunomodulatory agents, pregnancy, and breastfeeding.
Safety laboratory measures of haematological indices, liver function, thyroid-stimulating hormone, urea, creatinine, calcium, lipid levels, immunoglobulin levels, and urine analysis (pH, blood, protein, albumin/creatinine ratio, urine β-2-microglobulin, and cystatin-C) were performed at baseline, 4, 9, 14, 20, and 52 weeks. Serum and urine gold concentrations were measured at baseline, 1 day after the first and third dose, and at 9, 14, 20, and 52 weeks. The planned duration of the study was initially 20 weeks, but this was later extended to 52 weeks to continue safety follow-up of the participants due to a persistent local response to the intradermal injection (as described below). Skin changes at the injection site were followed up beyond 52 weeks in some individuals in the clinical care setting (12–24 months).
All subjects were observed for 6 h after their first dose/injection. The first subject was dosed >24 h before subsequent subjects as per sentinel dosing strategy. If no serious adverse events were observed after the first dose, all subjects were observed for a minimum of 1 h after each subsequent injection, with the plan to extend the observation period if there were any signs of hypersensitivity. All local and systemic reactions were documented. Safety data were under regular review by an independent Data Safety Monitoring Board (DSMB).
The primary end-point was an assessment of the safety of C19-A3 GNP administration; secondary end-points were assessments of changes in (i) C-peptide secretion at weeks 14 and 52, compared to baseline, assessed by a mixed-meal tolerance test (MMTT) and a stimulated urine C-peptide test, (ii) glycaemic control assessed by glucose profiles, insulin requirements, and HbA1c at weeks 14 and 52, compared with baseline, (iii) level or quality of lymphocyte biomarkers of β-cell-specific immune response, and (iv) level or quality of islet cell autoantibody biomarkers of β-cell-specific immune response.
Ethics statement
This study was carried out with the approval of the UK Research Ethics Service, Swedish Regional Ethical Review Board (RERB), UK Medicines and Healthcare products Regulatory Agency (MHRA) for Clinical Trial Authorisation, and Swedish Medical Products Agency (MPA). Written informed consent was obtained from all participants. The trial was conducted in compliance with the principles of the Declaration of Helsinki (1996) and the principles of Good Clinical Practice and in accordance with all applicable regulatory requirements including but not limited to the Research Governance Framework and the Medicines for Human Use (Clinical Trial) Regulations 2004, as amended in 2006. Further details are available at https://clinicaltrials.gov/.
Assays
Ensure Plus® [Abbott Nutrition, Maidenhead, UK; 6 ml/kg (max 360 ml)] was used as a mixed-meal stimulant of β-cell production, in both the standard MMTT and the assessment of stimulated urine C-peptide/creatinine ratio as described previously [41, 42]. MMTTs were carried out after an overnight fast at 0, 14, and 52 weeks. Serum samples for C-peptide and glucose were collected at −10, 0, 15, 30, 60, 90, and 120 min. Urine samples were collected from the second void in the morning (before MMTT) and 120 min after the MMTT (mixed-meal-urine C-peptide/creatinine ratio) with no urine loss in between.
Urine samples were collected in boric acid containers (Sterilin; Thermo Scientific, Newport, UK) and transported to a laboratory at ambient temperature within 72 h. If not assayed within 72 h of collection, they were stored at −80°C for up to 14 days. Serum samples were stored at −20°C and transported on dry ice in batches. Urine C-peptide level was measured in samples diluted 1:10, using an enzyme-linked immunosorbent assay (10-1136-01; Mercodia, Uppsala, Sweden). The detection limit for the C-peptide assay was 0.025 nmol/l, with intra- and inter-assay coefficients of variation of <5% and <5%, respectively. Urine creatinine was assayed using a colorimetric method (Jaffe reaction; CR8316; Randox Ltd, London, UK). The detection limit, and intra- and inter-assay coefficients of variation were 100 μmol/l, < 4% and <6%, respectively. Results were expressed as urine C-peptide/creatinine ratio (nmol/mmol). Serum C-peptide was measured using an immunochemiluminometric assay (IV2-004, Invitron, Monmouth, UK). The detection limit, and intra- and inter-assay coefficients of variation were 0.005 nmol/l, <5% and <8%, respectively.
Anti-glutamic acid decarboxylase antibody (GADA), anti-insulinoma-associated antigen 2 (IA-2A), and anti-Zinc transporter 8 (ZnT8A) were measured by enzyme-linked immunosorbent assay (GDE/96, IAE/96/2, ZnT8/96; RSR Ltd., Cardiff, UK) according to the manufacturer’s instructions. Positive cut-off values were ≥5, ≥7.5, and ≥15 U/ml for GADA, IA-2A, and ZnT8A, respectively. The detection limit for the GADA was 0.57 U/ml, for IA-2A was 1.25 U/ml, and for ZnT8A was 1.2 U/ml.
The gold concentration of the IMP was measured by Midatech Pharma Plc using microwave plasma atomic emission spectroscopy (MP-AES) and inductively coupled plasma-mass spectrometry (ICP-MS). Ligand ratios and purity of the IMP were measured by nuclear magnetic resonance (1H-NMR). Quantification of peptide bound to the GNP was performed using liquid chromatography–mass spectrometry (LC–MS). Particle hydrodynamic size and ζ potential were measured by dynamic light scattering (DLS) and particle diameter by transmission electron microscopy (TEM).
The concentration of gold in serum and urine samples was measured by ICP-MS, using a methodology developed and validated by Midatech Pharma Plc. The ICP-MS was a Perkin Elmer NEXION 300× instrument, equipped with the software NEXION (version 1.4). Serum and urine samples were digested with tetramethylammonium hydroxide (TMAH) solution, using iridium as an internal standard. Standards and control standards were prepared in the same way as the samples, using foetal bovine serum as an organic matrix. Samples were bracketed by control standards at low and high gold concentration. The concentration of gold in serum and urine was calculated interpolating in a simple-linear through-zero calibration curve (ranging from 0.1 to 100 ng/ml). Detection limit was 0.2 ng/ml and quantitation limit was 0.6 ng/ml.
Glucose variability was assessed by using a Dexcom Platinum G4 continuous glucose monitor (CGM). Recordings were taken for at least 72 h before study visits at weeks 0, 14, and 52.
Punch skin biopsies of the local area were performed under aseptic conditions and under local anaesthetic (Lidocaine Hydrochloride Injection BP 2%, w/v), using a 6-mm sterile disposable biopsy punch. Following the biopsy, samples were immediately placed in 10% formalin and transported to the laboratory.
Automatic tissue processing (Excelsior AS) and staining (Leica Autostainer) produced Haematoxylin & Eosin-stained tissue sections for assessment. Immunohistochemistry for CD2, CD3, CD4, CD8, CD20, CD79a, CD68, CD1a, and Ki67 was performed (Ventana Benchmark Ultra stainer).
The distribution of the gold was assessed using light microscopy of skin sections by catalytic deposition of silver. Dewaxed and rehydrated sections were incubated in Newman and Jasani’s physical developer [43]. Sections were then counterstained with Nuclear fast red + picro-methyl blue/light green [44]. The presence of elemental gold was confirmed on reprocessed samples using a transmission electron microscope (TEM; Philips CM12) fitted with an energy dispersive X-ray spectrometer (EDAX).
Gold hypersensitivity was assessed by using an epicutaneous patch test. Aqueous gold sodium thiosulphate (concentration range 2.0–0.0002%, w/v) and gold sodium thiosulphate in petrolatum 2% w/w were applied by using Finn Chambers (Chemotechnique Diagnostics, Sweden) to the skin on the participant’s back. The patches were removed after 48 h and the test was read after a further 48 h.
Details of immunological assays will be described in a separate publication (Hanna S.J. and are not part of this manuscript.
Statistical analysis
Data are expressed as mean ± SD and median and interquartile range. A Wilcoxon signed-rank test was used to test the significance of the change in relation to the baseline value. Differences were considered significant if the P-value was <0.05. GraphPad PRISM version 9.0 for Macintosh was used for the analysis.
The area under the curve (AUC) was calculated using the trapezoidal method, not adjusted for baseline C-peptide but normalised for the 120-min period of the standard MMTT using the serum C-peptide value at each time point.
The insulin dose-adjusted HbA1c (IDAA1c) was used as a surrogate measure of β-cell function. It examines the combined impact of changes in HbA1c and insulin usage on metabolic control. Levels ≤9 are considered favourable. It was calculated according to the formula: HbA1c (%) + [4 × insulin dose (units per kg per 24 h)] [45].
Safety assessments (i.e. safety blood and urine tests and IMP-related adverse events during the course of the study) were counted in terms of both the number of events and the number of participants. Injection site reactions were described verbally and photographed.
Results
Study enrolment and study participants
Twenty-four participants attended a screening visit. Participants who did not have either the HLA-DRB1∗0401 genotype (N = 12), stimulated serum/urine C-peptide below the defined threshold (N = 2) or both (N = 4) were excluded (Fig. 1a).
A total of six participants were enrolled in the study and received the first injection. Four participants received all three doses as planned. Two participants received only the first dose. One of them withdrew from further assessments due to competing time commitments and was withdrawn from further analysis, whilst another did not receive the second and third doses because of the decision to halt further drug administration. The latter participant remained in the study and attended all planned visits and assessments. A total of five participants were included in the final analysis.
The study was initially temporarily halted to obtain further information and expert advice about a delayed local reaction at the injection site that was observed in all participants (as described below). The study was subsequently terminated before recruitment of the planned eight participants was completed because the study was considered to have had achieved its primary aim, i.e. to assess the safety and tolerability of the IMP.
Mean participant age on entry was 28.00 ± 7.92 years [18–37]. Their age at diagnosis of T1D was 27.00 ± 10.12 years with a mean diabetes duration of 32.20 ± 35.39 months (Supplementary Table S1). Two out of the five participants were female, and all were of white ethnicity.
All participants were autoantibody-positive defined by being positive to at least one of GADA, IA-2A, or ZnT8A.
Evaluating changes in C-peptide levels
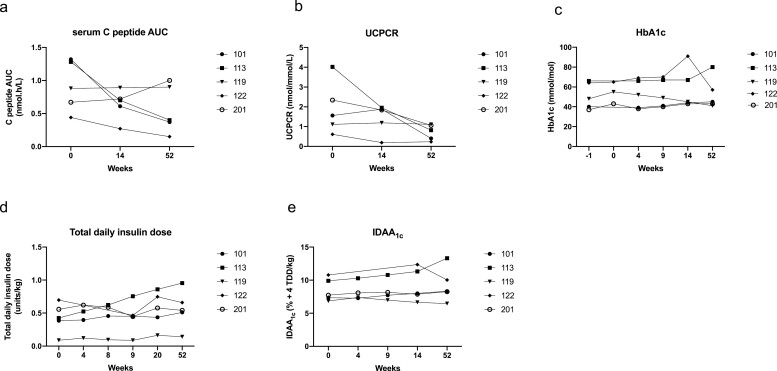
Median serum C-peptide AUC at baseline was 0.88 (0.56–1.30) nmol h/l. There was no significant difference in median serum C-peptide AUC at week 14 (0.70 (0.44–0.80) nmol h/l) and week 52 (0.40 (0.26–0.95) nmol h/l) in comparison to baseline. The serum C-peptide remained stable in two out of five participants (Fig. 2a, Supplementary Table S3).
Figure 2.
Effect of C19-A3 GNP on β-cell function and metabolic parameters. (a) Serum C-peptide expressed as area under the curve (AUC) over 120 min after mixed-meal challenge; (b) urine C-peptide to creatinine ratio (UCPCR) 2 h after mixed meal; (c) HbA1c; (d) median total daily insulin dose recorded over 3 days before the study visit and normalised for body weight; (e) insulin dose-adjusted A1c (IDAA1c) calculated according to the formula: HbA1c (%) + [4× insulin dose (units per kg per 24 h)]. Each line represents an individual participant with an assigned study number as shown in the legend.
Median UCPCR at baseline was 1.56 (0.86–3.18) nmol/mmol. There was no significant difference in median UCPCR at week 14 (1.84 (0.69–1.91) nmol/mmol) and week 52 (0.82 (0.31–1.08) nmol/mmol) in comparison to baseline. It remained stable in three out of five participants (Fig. 2b, Supplementary Table S3).
Evaluating glycaemic control
Median HbA1c at baseline was 48.0 (38.5–65.0) mmol/mol. There was no significant difference at week 4 (52.0 (38.5–67.5) mmol/mol), week 9 (49.0 (40.5–68.5) mmol/mol), week 14 (45.0 (43.5–79.0) mmol/mol), and week 52 (45.0 (42.0–68.5) mmol/mol) in comparison to baseline (Fig. 2c, Supplementary Table S3).
Median insulin dose at baseline was 0.43 (0.24–0.63) units/kg. It remained at similar levels at weeks 4, 9, and 14 (0.46 (0.19–0.60) units/kg, 0.52 (0.18–0.61) units/kg, and 0.45 (0.27–0.61) units/kg, respectively. The observed increase to 0.58 (0.30–0.80) units/kg at week 20 and to 0.54 (0.33–0.81) at week 52 was not statistically significant in comparison to baseline (Fig. 2d, Supplementary Table S3).
Median IDAA1c at baseline was 7.72 (7.10–10.34). It remained unchanged at weeks 4 and 9 (7.73 (7.31–9.74)) and (7.94 (7.17–10.13)), respectively. The observed increase to 8.00 (7.27–11.84) at week 14 and to 8.34 (7.37–11.68) at week 52 was not statistically significant in comparison to baseline (Fig. 2e, Supplementary Table S3).
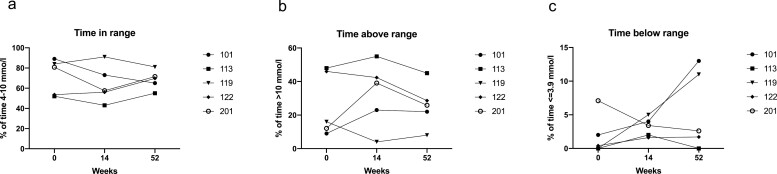
At the start of the study, glucose levels were in range (glucose between 4 and 10 mmol/l; measured by CGM) 80.80% (52.80–86.50%) of the recorded period (at least 72 h). This did not change significantly when assessed at 14 and 52 weeks (57.50 (49.50–82.00)% and 69.70 (60.00–76.25)%, respectively) – Fig. 3a, Supplementary Table S3.
Figure 3.
Effect of C19-A3 GNP on glucose variability. (a) Time in range (the % of time participants recorded a blood glucose level of 4–10 mmol/l); (b) time above range (the % of time participants recorded a blood glucose level of >10 mmol/l); (c) time below range (the % of time participants recorded a blood glucose level of <4 mmol/l). Recordings were taken for at least 72 h before study visits. Each line represents an individual participant with an assigned study number as shown in the legend.
There was no significant difference in time spent above range (glucose >10 mmol/l) at week 14 (39.10 (13.50–48.70) %) or week 52 (25.90 (15.00–36.80) %) in comparison to baseline (16.00 (10.55–47.00) %) – Fig. 3b, Supplementary Table S3.
There was also no significant difference in time spent below range (glucose <4 mmol/l) at week 14 (3.40 (1.80–4.50) %) and 52 (2.60 (0.85–12.00)) in comparison to baseline (0.40 (0–4.55) %) – Fig. 3c, Supplementary Table S3.
Evaluating gold serum concentration and excretion
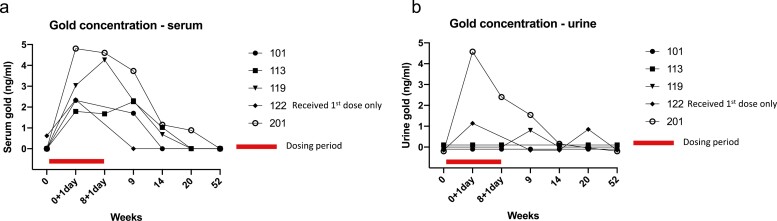
Serum gold concentrations increased in all participants after the first and third dose of C19-A3 GNP peptide. The decline in the gold serum concentrations was observed as early as week 9 (1 week after the third dose), although detectable levels were present in all participants at that point in time, with the exception of the participant who received the first dose only. A reduction in gold serum concentration became more evident at weeks 14 and 20, when only three and one participant(s), respectively, had detectable levels. Serum gold levels were undetectable at week 52 in all participants – Fig. 4a, Supplementary Table S4.
Figure 4.
Serum and urine gold concentration following administration of C19-A3 GNP. (a) Serum gold concentration; (b) urine gold concentration. Blood and urine samples for measurement of gold concentration were taken at baseline (week 0), one day after 1st dose (week 0 + 1 day) and one day after 3rd dose (week 8 + 1 day). Detection limit was 0.2 ng/ml and quantitation limit 0.6 ng/ml. Levels below detection and quantitation limit are presented as zero on the graph. Each line represents an individual participant with an assigned study number as shown in the legend.
Measurements of gold in the urine showed a less consistent pattern. An increase in urinary gold concentration after dosing was recorded in two of the five participants 1 day after the first dose, one of four participants 1 day after the third dose, and in two of the four participants 1 week after the third dose. Urinary gold urine levels were undetectable at week 52 in all participants – Fig. 4b, Supplementary Table S5.
Safety assessments in blood and urine tests
There were no clinically significant abnormalities in safety blood and urine tests and no IMP-related serious adverse events were reported during the course of the study. There was one serious adverse event requiring hospital admission, which was secondary to Campylobacter infection, which was considered unrelated to the IMP (Supplementary Table S6).
Participants also reported headache, mild upper respiratory tract infection symptoms, and nausea and vomiting during the study. None of the reported adverse events were considered to be directly related to the IMP (Supplementary Table S6).
Injection site reactions and gold hypersensitivity
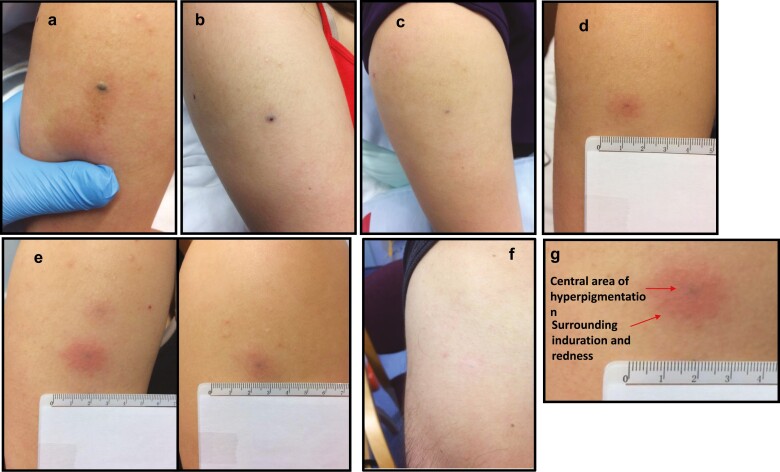
All participants developed an immediate localised skin reaction after all IMP injections. It consisted of an asymptomatic GNP-derived dark-coloured central area (3–4 mm) surrounded by a circular erythematous area (diameter of 2–3 cm), as illustrated in Fig. 5a–g. Erythema disappeared after 45–60 min of close observation, but the dark central area persisted and reduced in size to 1–2 mm by the following day.
Figure 5.
Skin changes after intradermal injection of C19-A3 GNP. (a) Immediately after injection; (b) 5 min after injection; (c) 24 h after injection; (d) 30 days after injection; (e) 2 months after injection (upper left), 1 month after injection (right) and 7 days after injection (lower left); (f) 20 months after injection; (g) close-up of the injection site showing central area of hyperpigmentation and surrounding induration and redness.
Subsequently, delayed skin changes over the injection site were observed. The change consisted of a small central area of GNP-derived pigmentation surrounded by an asymptomatic circular erythematous induration. Induration initially reached 2–3 cm in diameter that reduced to approximately 1 cm during the follow-up period. These delayed reactions appeared 17.4 ± 2.3 days after the first injection, but much sooner, i.e. 1–3 days after the second and third injection. This was consistent in all participants. The skin changes significantly faded over the course of the study, although they were still visible at the end of the observation period (12–24 months depending on the participant) – Fig. 5a–g.
Four participants who received the IMP injection agreed to have gold hypersensitivity testing. All had positive reactions with epicutaneous patch testing to gold sodium thiosulphate 2% in petrolatum.
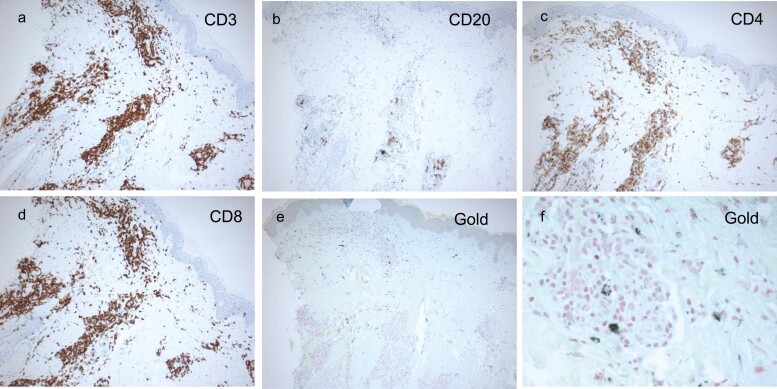
A skin biopsy of the injection site was performed on two participants. Haematoxylin & eosin-stained tissue sections showed a normal epidermis with perivascular inflammation in the upper- and mid-dermis. The inflammatory infiltrate was composed of lymphocytes and histiocytes, but no granulomas were present. Immunohistochemistry showed the infiltrate to be mainly CD3-positive T-cell lymphocytes (equal distribution of CD4+ and CD8+) with small numbers of CD20-positive B-cell lymphocytes. There was also evidence of intradermal gold retention – Fig. 6a–f.
Figure 6.
Histopathology and immunohistochemistry staining of the punch biopsies of the injection sites. (a) Anti-CD3 staining; (b) anti-CD20 staining; (c) anti-CD4 staining; (d) anti-CD8 staining; (e) and (f) gold staining.
Discussion
This study has explored the concept of enhanced antigen-specific immunotherapy by conjugating proinsulin peptide C19-A3 which has a known favourable immunomodulating profile [28] to very small gold nanoparticles to target antigen delivery to potentially tolerogenic DCs in the skin.
This first-in-human study was designed to assess the safety and tolerability of the new construct. In general, dosing was well tolerated by patients. The study was not powered to assess effects on β-cell preservation, although there was no evidence of accelerated β-cell function decline in the small number of participants. Participants were not recruited immediately after diagnosis of T1D as these patients are often recruited to other immunomodulatory trials in T1D. However, the requirement for a participant to have a threshold C-peptide value ensured they had a residual β-cell function at the time of their enrolment in the study. Two participants maintained stable levels of stimulated C-peptide and three had an IDAAC1 level in the favourable range (<9) for the duration of the trial [45]. However, in at least one participant stable C-peptide levels can be explained by the stage of their diabetes, i.e. a slow natural decline in C-peptide in that phase of the disease was expected. Others showed moderate decline in endogenous insulin production, which again may be consistent with the natural progression of T1D during the early years of the disease (three out of five participants), Supplementary Table S2 [46]. Importantly, insulin requirements of most participants remained low (<0.8 units/kg of body weight) throughout the study and there was reasonably good glycaemic control with median HbA1c of 45.00 (42.0–68.5) mmol/mol and glucose levels, recorded at the time of the last visit, that was maintained within expected range for 69.7% (60.0–76.2%) of the time (Figs. 2 and 3).
There was no evidence of systemic hypersensitivity and no IMP-related serious adverse effects were observed during the study. In addition, there was no evidence of systemic gold retention or effect as evidenced by normalisation of serum and urine gold concentration (Fig. 4, Supplementary Tables S4 and S5) and normal safety tests including renal and liver function tests.
All participants developed a delayed local skin reaction at the site of each injection (Fig. 5). It appeared 1–3 weeks after the first injection, 1–3 days after the second and third injection, and persisted, gradually fading over 12–24 months. Histopathological findings indicated retention of gold in the dermis (Fig. 6e and f) with B- and T-cell infiltrate in distribution and quantity atypical to normal skin, but not consistent with foreign body granuloma (Fig. 6). Immunological analysis of this infiltrate and its antigen specificity (gold vs. C19-A3 peptide) will be reported separately (Hanna S.J.). However, the observation of the prolonged retention of the GNP (and possibly the whole construct, including peptide) in the skin is a unique outcome of this study that opens a number of therapeutic approaches, including the creation of a depot with the potential to create a sustained immunomodulating environment in the skin. In this example, the addition of tolerogenic cargo could facilitate a favourable effect on autoimmune processes and β-cell function.
To assess the possibility of induced gold hypersensitivity, participants had a skin patch hypersensitivity test to gold thiosulphate which came back positive in all tested subjects. Gold thiosulphate was chosen as it is the antigen routinely used in standard clinical practice for patch testing to detect gold hypersensitivity. Contact allergy can occur in routinely patch-tested patients, but the clinical relevance of this is unclear and often not demonstrated, as a systemic hypersensitivity rarely, if ever, develops [47, 48]. There is a potential cosmetic drawback of permanent or semi-permanent skin changes. These could be practically overcome in future studies by choosing less exposed areas for injection sites and they may be more or less visible in different skin types. The occurrence of injection site reactions was reviewed by the DSMB who considered that events did not adversely change the risk/benefit ratio and did not constitute a safety issue.
We are of the opinion that benefits of this approach (well-tolerated, minimally invasive intradermal administration that could be modified to self-administration in the future; potential for creating a sustained tolerogenic immunomodulating environment in the skin; no systemic gold retention or effects) outweigh the risks (possible induction of gold hypersensitivity with unknown clinical significance and no evidence of systemic hypersensitivity; unfavourable cosmetic effect).
In summary, allowing for the limitation of the small number of participants, intradermal administration of C19-A3 GNP constructs appears safe and well tolerated in people with T1D. The associated prolonged skin retention after intradermal administration offers a number of possibilities to enhance its tolerogenic potential in T1D, which should be explored in future studies.
Supplementary Material
Acknowledgements
We thank R. Minguez and I. Fernandez for IMP manufacture (Midatech Pharma Plc, Derio, Spain), J. Barrenetxea for MP-AES and ICP-MS analyses, N. Intxausti for DLS, LC–MS analysis and KR Patel (Midatech Pharma Plc) for LC–MS analysis and Nadim Bashir (Swansea Trials Unit) for contributing to data management tool development. The Editor-in-Chief and handling editor, Tim Elliott, would like to thank the reviewer Parth Narendran and an anonymous reviewer for their contribution to the publication of this article.
Glossary
Abbreviations
- ACR
Albumin/creatinine ratio
- GADA
Anti-glutamic acid decarboxylase antibody
- IA-2A
Anti-insulinoma associated antigen 2
- ZnT8A
Anti-zinc transporter 8
- ASI
Antigen-specific immunotherapy
- AUC
Area under the curve
- CGM
Continuous glucose monitor
- DSMB
Data Safety Monitoring Board
- DLS
Dynamic light scattering
- GNPs
Gold nanoparticles
- ICP-MS
Inductively coupled plasma-mass spectrometry
- IDAA1c
Insulin dose-adjusted HbA1c
- IMP
Investigational medicinal product
- LC–MS
Liquid chromatography–mass spectrometry
- MPA
Medical products agency
- MHRA
Medicines and healthcare products regulatory Agency
- MP-AES
Microwave plasma atomic emission spectroscopy
- MMTT
Mixed-meal tolerance test
- 1H-NMR
Nuclear magnetic resonance
- PES
Poly ether sulfone
- RERB
Regional Ethical Review Board
- TMAH
Tetramethylammonium hydroxide
- TEM
Transmission electron microscopy
- T1D
Type 1 diabetes
- UCPCR
Urine C-peptide to creatinine ratio
Author contributions
D.T.: conception and design of work; acquisition, analysis, and interpretation of data; drafting and revising the manuscript. M.A.Mc.A.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript. J.B.: acquisition, analysis, and interpretation of data; revising the work. A.B.: acquisition, analysis, and interpretation of data; revising the manuscript. K.R.T.: acquisition, analysis, and interpretation of data; revising the manuscript. I.P.: acquisition, analysis, and interpretation of data; revising the manuscript. E.K.: interpretation of data; revising the manuscript. Y.L.: interpretation of data; revising the manuscript. M.D.: acquisition, analysis, and interpretation of data; revising the manuscript. S.A.C.: conception and design of work; analysis and interpretation of data; revising the manuscript. J.C.B.: conception and design of work; analysis and interpretation of data; revising the manuscript. C.v.R.: acquisition, analysis, and interpretation of data; revising the manuscript. A.H.: acquisition and interpretation of data; revising the manuscript. R.S.: acquisition and interpretation of data; revising the manuscript. M.A.A.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript. S.D.L.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript. G.D.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript; W.Y.C.: analysis and interpretation of data; revising the manuscript. G.H.: conception and design of work; analysis and interpretation of data; revising the manuscript. K.M.: acquisition, analysis, and interpretation of data; revising the manuscript. J.R.I.: acquisition, analysis and interpretation of data; revising the manuscript. M.M.U.C.: acquisition, analysis, and interpretation of data; revising the manuscript. F.S.W.: conception and design of work; analysis and interpretation of data; revising the manuscript. R.C.: acquisition, analysis, and interpretation of data; revising the manuscript. C.M.D.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript. J.L.: conception and design of work; acquisition, analysis, and interpretation of data; revising the manuscript. Authors approved the final version to be published and are in agreement to be accountable for all aspects of this work.
Funding
This work has been funded through the EE-ASI (The Enhanced Epidermal Antigen Specific Immunotherapy Against Type 1 Diabetes) European research network (Collaborative Project) supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (grant no. N 305305).
Conflict of interest
J.R.I. is Editor-in-Chief of the British Journal of Dermatology. He is a Consultant for UCB Pharma, Novartis, Boehringer Ingelheim and ChemoCentryx and participated in Advisory Boards for Kymera Therapeutics and Viela Bio, all in the field of hidradenitis suppurativa.
Y.L. is the CEO of NanoPass Technologies Ltd. EK is the Medical Director of NanoPass Technologies Ltd. M.M.U.C. is clinical lead for Dermatology e-learning for Health (HEE) for British Association of Dermatologists.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Foster NC, Beck RW, Miller KM.et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skyler JS. Primary and secondary prevention of Type 1 diabetes. Diabet Med. 2013;30(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dayan CM, Korah M, Tatovic D.et al. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet 2019;394:1286–96. [DOI] [PubMed] [Google Scholar]
- 4. Mallone R, Brezar V, Boitard C.. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin Dev Immunol 2011;2011:513210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian J, Kaufman DL.. Antigen-based therapy for the treatment of type 1 diabetes. Diabetes 2009;58:1939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Herrath M, Peakman M, Roep B.. Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol 2013;172:186–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkinson MA, Roep BO, Posgai A.et al. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roep BO, Wheeler DCS, Peakman M.. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 2019;7:65–74. [DOI] [PubMed] [Google Scholar]
- 9. Ludvigsson J. Autoantigen treatment in type 1 diabetes: unsolved questions on how to select autoantigen and administration route. Int J Mol Sci. 2020;21(5):1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diabetes Prevention Trial—Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–91. [DOI] [PubMed] [Google Scholar]
- 11. Orban T, Farkas K, Jalahej H.et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun 2010;34:408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludvigsson J, Faresjö M, Hjorth M.et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–20. [DOI] [PubMed] [Google Scholar]
- 13. Wherrett DK, Bundy B, Becker DJ.et al.; Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson J, Krisky D, Casas R.et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012;366:433–42. [DOI] [PubMed] [Google Scholar]
- 15. Chaillous L, Lefèvre H, Thivolet C.et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet 2000;356:545–9. [DOI] [PubMed] [Google Scholar]
- 16. Skyler JS, Krischer JP, Wolfsdorf J.et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial—type 1. Diabetes Care 2005;28:1068–76. [DOI] [PubMed] [Google Scholar]
- 17. Näntö-Salonen K, Kupila A, Simell S.et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–55. [DOI] [PubMed] [Google Scholar]
- 18. Fourlanos S, Perry C, Gellert SA.et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 2011;60:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weaver DJ Jr, Liu B, Tisch R.. Plasmid DNAs encoding insulin and glutamic acid decarboxylase 65 have distinct effects on the progression of autoimmune diabetes in nonobese diabetic mice. J Immunol 2001;167:586–92. [DOI] [PubMed] [Google Scholar]
- 20. Johnson MC, Wang B, Tisch R.. Genetic vaccination for re-establishing T-cell tolerance in type 1 diabetes. Hum Vaccin 2011;7:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walter M, Philotheou A, Bonnici F.et al.; NBI-6024 Study Group. No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 2009;32:2036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathieu C, Gillard P.. Arresting type 1 diabetes after diagnosis: GAD is not enough. Lancet 2011;378:291–2. [DOI] [PubMed] [Google Scholar]
- 23. Adorini L, Penna G.. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol 2009;188:251–73. [DOI] [PubMed] [Google Scholar]
- 24. Ali MA, Thrower SL, Hanna SJ.et al. Topical steroid therapy induces pro-tolerogenic changes in Langerhans cells in human skin. Immunology 2015;146:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boks MA, Kager-Groenland JR, Haasjes MS.et al. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction – a comparative study of human clinical-applicable DC. Clin Immunol 2012;142:332–42. [DOI] [PubMed] [Google Scholar]
- 26. Hannelius U, Beam CA, Ludvigsson J.. Efficacy of GAD-alum immunotherapy associated with HLA-DR3-DQ2 in recently diagnosed type 1 diabetes. Diabetologia 2020;63:2177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thrower SL, James L, Hall W.et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol 2009;155:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alhadj Ali M, Liu YF, Arif S.et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017;9(402):eaaf7779. [DOI] [PubMed] [Google Scholar]
- 29. de Araújo RF Júnior, de Araújo AA, Pessoa JB.et al. Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharmacol Rep 2017;69:119–29. [DOI] [PubMed] [Google Scholar]
- 30. Dul M, Nikolic T, Stefanidou M.et al.; EE-ASI Consortium. Conjugation of a peptide autoantigen to gold nanoparticles for intradermally administered antigen specific immunotherapy. Int J Pharm 2019;562:303–12. [DOI] [PubMed] [Google Scholar]
- 31. Merad M, Ginhoux F, Collin M.. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 2008;8:935–47. [DOI] [PubMed] [Google Scholar]
- 32. Steinman RM, Hawiger D, Nussenzweig MC.. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685–711. [DOI] [PubMed] [Google Scholar]
- 33. Klechevsky E, Banchereau J.. Human dendritic cells subsets as targets and vectors for therapy. Ann N Y Acad Sci 2013;1284:24–30. [DOI] [PubMed] [Google Scholar]
- 34. Chu CC, Ali N, Karagiannis P.et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012;209:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh RK, Malosse C, Davies J.et al. Using gold nanoparticles for enhanced intradermal delivery of poorly soluble auto-antigenic peptides. Nanomedicine 2021;32:102321. [DOI] [PubMed] [Google Scholar]
- 36. Levin Y, Kochba E, Hung I.et al. Intradermal vaccination using the novel microneedle device MicronJet600: Past, present, and future. Hum Vaccin Immunother 2015;11:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levin Y, Kochba E, Shukarev G.et al. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine 2016;34:5262–72. [DOI] [PubMed] [Google Scholar]
- 38. Carter D, van Hoeven N, Baldwin S.et al. The adjuvant GLA-AF enhances human intradermal vaccine responses. Sci Adv 2018;4:eaas9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beals CR, Railkar RA, Schaeffer AK.et al. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella-zoster virus vaccine: an exploratory, randomised, partly blinded trial. Lancet Infect Dis 2016;16:915–22. [DOI] [PubMed] [Google Scholar]
- 40. Hung IF, Yap DY, Yip TP.et al. A double-blind randomized phase 2 controlled trial of intradermal hepatitis B vaccination with a topical Toll-like receptor 7 agonist imiquimod, in patients on dialysis. Clin Infect Dis. 2021;73:e304–11. [DOI] [PubMed] [Google Scholar]
- 41. Greenbaum CJ, Mandrup-Poulsen T, McGee PF.et al.; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tatovic D, Luzio S, Dunseath G.et al.; MonoPepT1De Study Group. Stimulated urine C-peptide creatinine ratio vs serum C-peptide level for monitoring of β-cell function in the first year after diagnosis of Type 1 diabetes. Diabet Med 2016;33:1564–8. [DOI] [PubMed] [Google Scholar]
- 43. Newman GR, Jasani B.. Silver development in microscopy and bioanalysis: a new versatile formulation for modern needs. Histochem J 1998;30:635–45. [DOI] [PubMed] [Google Scholar]
- 44. Dhanjal TS, Lellouche N, von Ruhland CJ.et al. Massive accumulation of myofibroblasts in the critical isthmus is associated with ventricular tachycardia inducibility in post-infarct swine heart. Jacc Clin Electrophysiol 2017;3:703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mortensen HB, Hougaard P, Swift P.et al.; Hvidoere Study Group on Childhood Diabetes. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greenbaum CJ, Beam CA, Boulware D.et al.; Type 1 Diabetes TrialNet Study Group. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruze M, Edman B, Björkner B.et al. Clinical relevance of contact allergy to gold sodium thiosulfate. J Am Acad Dermatol 1994;31:579–83. [DOI] [PubMed] [Google Scholar]
- 48. Chen JK, Lampel HP.. Gold contact allergy: clues and controversies. Dermatitis 2015;26:69–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.