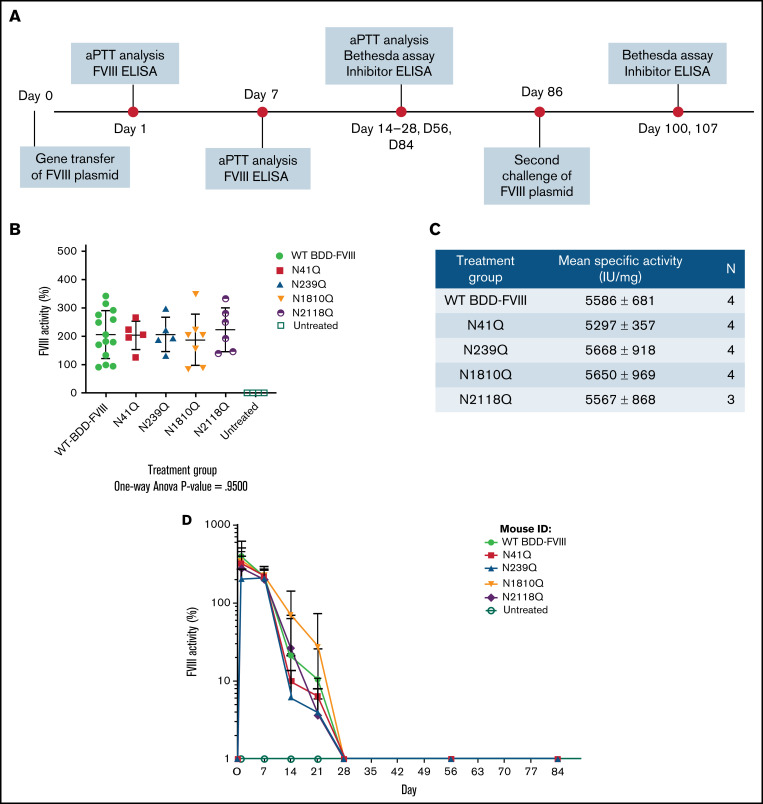
Figure 2.
In vivo experimental layout and comparison of activities of mutated FVIII variants and WT BDD-FVIII. Groups of HA mice were injected hydrodynamically with plasmids encoding WT BDD-FVIII and 4 N-glycosylation site mutated FVIII variants, respectively. (A) The experimental schedule of first and second challenges of FVIII plasmids via hydrodynamic injections and periodic blood collection for aPTT analysis, Bethesda assay, and inhibitor enzyme-linked immunosorbent assay (ELISA). (B) FVIII activities in plasma by aPTT at 1 week after plasmid injection. P = .95 calculated using 1-way analysis of variance among all plasmid treated groups. Untreated mice were used as negative controls. (C) Specific activities of each FVIII variant compared with WT BDD-FVIII. Antigen levels of FVIII were evaluated using a FVIII-specific ELISA. N indicates the number of animals per group. (D) FVIII activities evaluated over time. WT BDD-FVIII: N = 14; N41Q: N = 5; N239Q: N = 5; N1810Q: N = 7; N2118Q: N = 6; untreated: N = 4. Experiments for each group were repeated at least 3 times. Data are presented as averages from repeated experiments, with error bars indicating standard deviation.