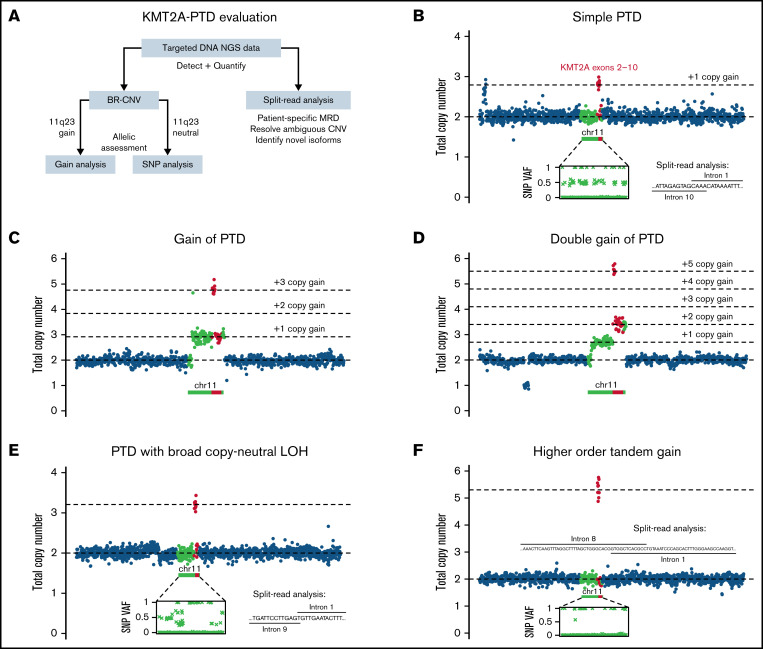
Figure 1.
Patterns of KMT2A-PTD allelic states from targeted DNA NGS data. (A) Outline of strategy for detection, quantification, and allelic characterization of KMT2A-PTD. (B) Simple KMT2A-PTD cells harbor 1 wild-type allele (1 copy KMT2A) and 1 PTD allele (2 copies of PTD exons and 1 copy of other exons) by definition, for a net +1 copy gain of PTD exons relative to diploid baseline. In bulk NGS data, simple KMT2A-PTDs are thus characterized by copy-neutral 3′ KMT2A, shallow gain of PTD exons (proportional to percent cells involved, ranging up to +1 at 100% clonality), and balanced heterozygous SNPs along 11q. This example had a bulk average of 2.79 total copies of exons 2 to 10 (or +1 × 0.79 change from diploid), indicating a simple KMT2A-PTD involving 79% of cells. Split reads also confirmed the PTD. (C) Complex KMT2A-PTD cells with gain of 11q23.3 from the PTD allele harbor an extra PTD (thus 2 extra copies of PTD exons, 1 extra copy of other exons) relative to a simple KMT2A-PTD (+1 copy gain of PTD exons), or net +3 copy gain of PTD exons and +1 copy gain of other exons relative to diploid. This example (P1) was consistent with 11q23.3 gain from the PTD allele involving 92% of cells. The PTD was cytogenetically cryptic as usual; however, the regional gain was characterized by karyotype as the broad distal duplication dup(11)(q13q25) and quantified at a similar level of 87% cells by KMT2A FISH. (D) Complex KMT2A-PTD cells with double gain of 11q23.3 from the PTD allele harbor 2 extra PTDs (thus 4 extra copies of PTD exons, 2 extra copies of other exons) relative to a simple KMT2A-PTD or net +5 copy gain of PTD exons and +2 copy gain of other exons relative to diploid. This example (P2) had a TP53 mutation, complex karyotype, and stepwise copy number profile over 11q, suggesting breakage-fusion-bridge cycles. The findings were consistent with a KMT2A-PTD subject to 2 sequential distal 11q gain events from the PTD allele occurring in 70% of cells. (E) Complex KMT2A-PTD cells with CN-LOH have 0 wild-type alleles and 2 PTD alleles, for a net change of +2 copy gain of PTD exons. This example (P5) had a normal karyotype, no copy number changes over chromosome 11 except for bulk average +1.2 copy gain of KMT2A exons 2 to 9, and allelic imbalance of heterozygous SNPs spanning 11q targets, thus indicating KMT2A-PTD with broad 11q CN-LOH. By contrast, prior studies have reported only monoallelic involvement in cytogenetically normal cases.5 This example was predicted to be a mixture of simple and complex KMT2A-PTDs based on VAFs of heterozygous SNPs relative to copy gain level of PTD exons. (F) Gains of more than +2 copies of PTD exons in the setting of baseline diploid KMT2A could not be explained solely by CN-LOH of a simple KMT2A-PTD. This example (P8) demonstrated bulk average +3.55 copies of KMT2A exons 2 to 8, broad CN-LOH of 11q, and no other copy number changes. The gain was entirely attributable to a single PTD mutant junction connecting intron 8 to intron 1, because split-reads yielded a similar estimate of +3.28 copies. This magnitude of gain in the context of a single mutant junction raised the possibility of episomal amplification whereas CN-LOH favored localization to chromosome 11; thus, one possibility might be intrachromosomal amplification from episomal re-integration, which has been described at other genomic loci.12,13 VAFs, variant allele fractions.