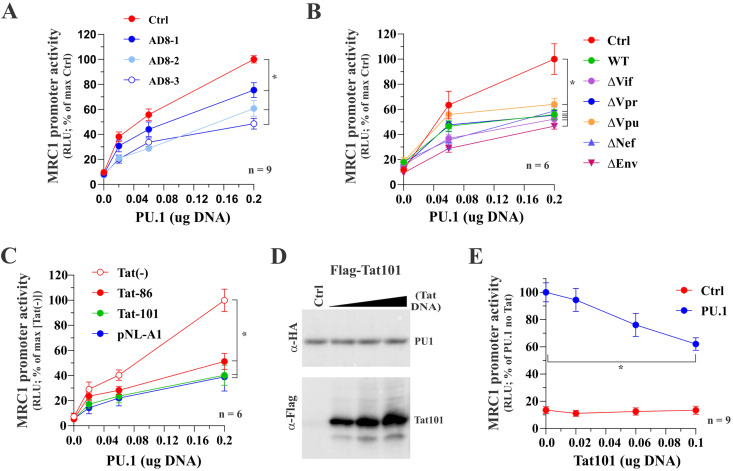
FIG 5.
Tat inhibits the PU.1-dependent activation of the hMRC1 promoter. HEK293T cells were plated into 24-well plates (5 × 104 cells/well) and transfected in triplicate with a total of 0.5 μg DNA mixture each. Luciferase production was determined 2 days posttransfection as described in Materials and Methods. (A) Full-length AD8 inhibits PU.1-mediated activation of the MRC1 promoter. Cells were transfected with pGL-hMRC1-Luc (0.1 μg) and increasing amounts of PU.1 (0.02 μg, 0.06 μg, and 0.2 μg) as well as pAD8 WT (AD8-1, 0.02 μg; AD8-2, 0.06 μg; AD8-3, 0.2 μg). The effect of PU.1 in the absence of AD8 was assessed in parallel (red circles). Mean and error bars representing SEM from nine independent transfections (n = 9) are shown. MRC1 promoter activity was statistically significantly different between the control sample and all three AD8-transfected samples at 0.2 μg PU.1 (indicated by an asterisk). (B) Inactivation of individual HIV-1 genes has no effect on the ability of AD8 to interfere with the function of PU.1. Cells were transfected with pGL-hMRC1-Luc (0.1 μg) and increasing amounts of PU.1 (0.06 μg, 0.2 μg) as well as 0.2 μg each of pAD8 WT or pAD8 mutants as indicated. A control lacking AD8 proviral DNA was included as reference (red circles). Mean and error bars representing SEM from six independent transfections (n = 6) are shown. The MRC1 promoter activity of all virus-transfected samples was statistically significantly different from the control sample at 0.2 μg PU.1 (indicated by an asterisk). (C) Tat expression is sufficient to inhibit PU.1 activity. Cells were transfected with pGL-hMRC1-Luc (0.1 μg) and increasing amounts of PU.1 (0.02 μg, 0.06 μg, and 0.2 μg) either in the absence of Tat [Tat(−)] or the presence of 0.1 μg of pTat86 or pTat101 or 0.2 μg of pNL-A1 DNA. Mean and error bars representing the SEM from six independent transfections (n = 6) are shown. MRC1 promoter activity of all three transfected samples was statistically significantly different from that of the [Tat(−)] at 0.2 μg PU.1 (indicated by an asterisk). (D) Tat does not affect the stability of PU.1 protein. HEK293T cells were transfected in 25-cm2 flasks with 2 μg of pcDNA-PU.1-HA and increasing amounts (1 μg, 2 μg, and 3 μg) of p3xFlag-Tat101. The total amount of transfected DNA was adjusted in all samples to 5 μg each using empty vector DNA as appropriate. Cells were collected 24 h later, and whole-cell extracts were subjected to immunoblot analysis using antibodies to HA (PU.1) and Flag (Tat101) epitope tags. Expression of PU.1 in the absence of Tat served as reference (Ctrl). (E) Inhibition of the hMRC1 promoter by Tat. Cells were transfected with pGL-hMRC1-Luc (0.1 μg) and increasing amounts of pTat101 (0, 0.02, 0.06, and 0.1 μg) either in the absence of PU.1 (Ctrl) or in the presence of 0.1 μg of PU.1 DNA (PU.1). Means and error bars representing SEM from nine independent transfections (n = 9) are shown. In the presence of PU.1, MRC1 promoter activity was statistically significantly different between 0 μg and 0.2 μg Tat (P < 0.001); this was not the case in the absence of PU.1 (P = 0.985). The slope of Tat on MRC1 promoter activity was significant only in the presence of PU.1, and the slope of the curve in each group was statistically significantly different from each other. For details of the statistical analyses, see Fig. S1 in the supplemental material.