FIG 9.
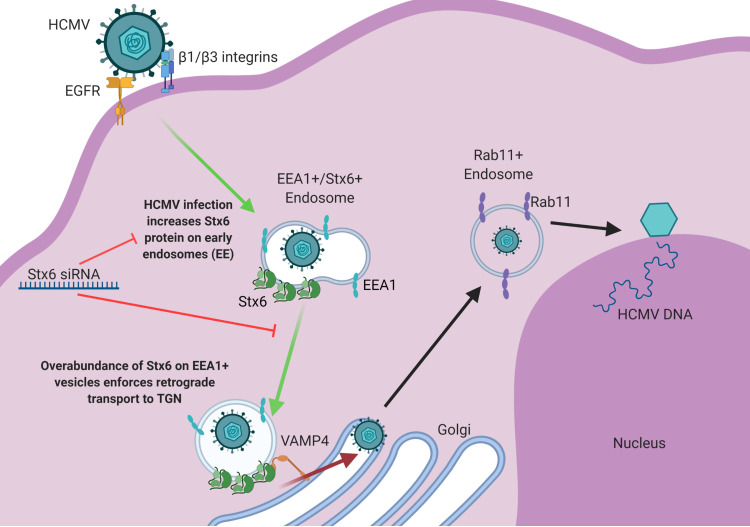
Model: HCMV infection upregulates Stx6, which facilitates viral retrograde transport to the TGN, and thereby nuclear translocation. HCMV infection of monocytes triggers a significant increase in Stx6 protein, which accumulates on early endosomes. At approximately 15–45 mpi, HCMV is taken into early endosomes. A portion of viral particles are taken into vesicles containing both EEA1 and Stx6. Overabundance of Stx6 enforces or biases retrograde transport of these virus-containing vesicles to the TGN. At the TGN, the virus-containing endosome, laden with Stx6, can bypass retrograde transport-limiting complexes and bind directly to cognate receptors at the TGN surface such as VAMP4. This Stx6-dependent interaction mediates membrane fusion between the virus-carrying vesicle and the TGN. Once in the lumen of the TGN, the viral particles then subsequently travel on to recycling endosomes and then to the nucleus where viral genome replication and subsequent productive infection can occur. Stx6 siRNA inhibits the HCMV-driven upregulation of Stx6, which consequently inhibits viral trafficking to the TGN – a necessary trafficking event prior to nuclear translocation in monocytes. Image created with BioRender.com.