FIGURE 5.
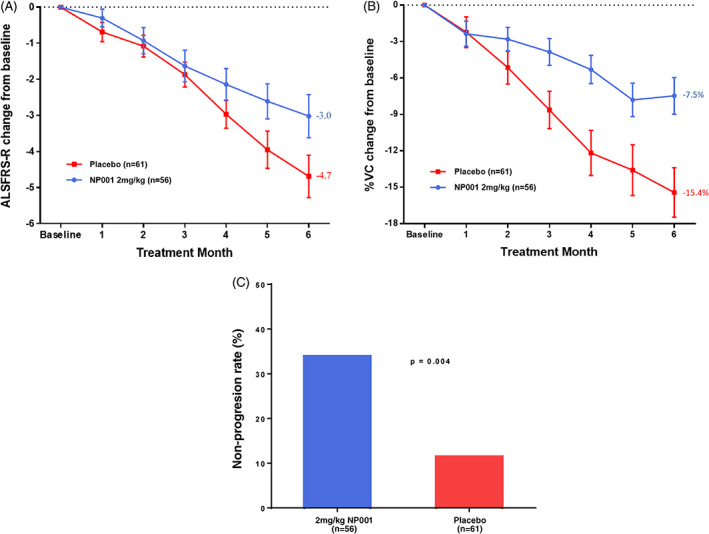
Post hoc analysis of NP001 efficacy in participants with plasma CRP > 1.13 mg/L and age between 40 and 65 y at baseline in phase 2A and 2B trials. A, Participants treated with NP001 experienced a slower decline in ALSFRS‐R score from baseline compared with placebo in those with plasma CRP > 1.13 mg/L and age between 40 and 65 y at baseline in phase 2A and 2B trials. ALSFRS‐R score change from baseline for participants treated with NP001 (n = 56) depicted in blue and compared with placebo group (n = 61) depicted in red. Bars represent mean of ALSFRS‐R score change from baseline ± SEM. The NP001 treatment group showed a 36% slower progression rate by the end of study (Wilcoxon test, p = .01). B, Percent change in vital capacity (VC) over 6 mo in participants on NP001 compared with placebo in those with plasma CRP > 1.13 mg/L and age between 40 and 65 y at baseline in phase 2A and 2B trials. Percent predicted VC change from baseline for participants treated with NP001 (n = 56) is depicted in blue and compared with the placebo group (n = 61) depicted in red. Bars represent mean of % predicted VC change from baseline ± SEM. Average %VC lost over the 6 mo of study: NP001: −7.5% (−1.3% per month); Placebo: −15.4% (−2.6% per month). The NP001 treatment arm lost 51% less respiratory function than the placebo arm by the end of study (Wilcoxon test, p < .001). C, NP001 treatment was associated with stabilized disease in participants over 6‐mo studies. Non‐progressors defined as having no decrease in ALSFRS‐R score from baseline to 6 mo, by treatment group, restricted to those with plasma CRP > 1.13 mg/L and age between 40 and 65 y at baseline in phase 2A and 2B trials. The proportion of non‐progressors (non‐progression rate) in 2 mg/kg NP001 treatment (19 out of 56) was significantly greater than that of the placebo group (7 out of 61) (Fisher exact test, p = .004). In participants with plasma CRP > 3 mg/L at baseline, the non‐progression rate for NP001 treated was 46% (13/28) versus 4.5% (1/22) in the placebo group (Fisher exact test, p = .001)
