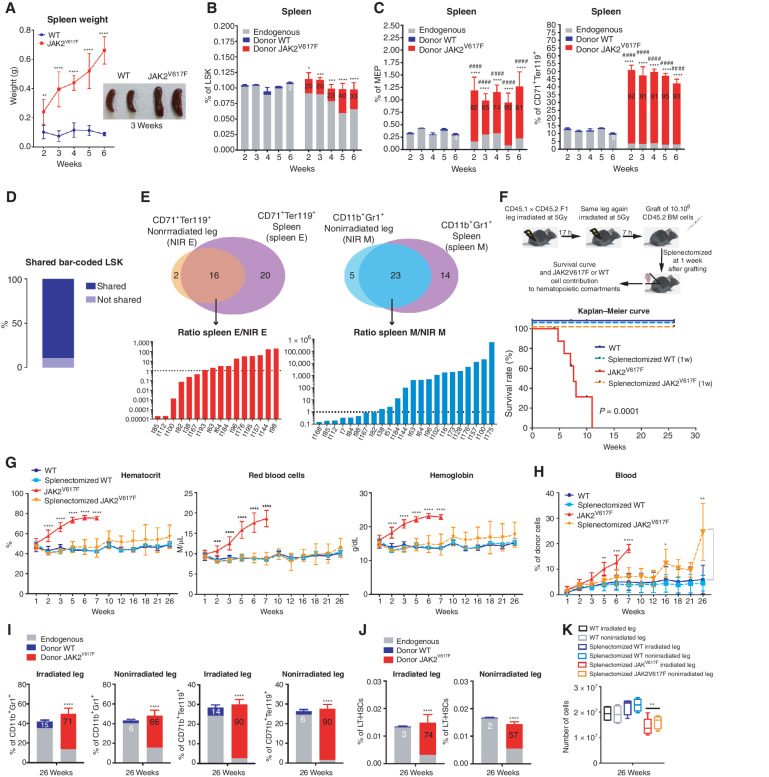
Figure 3.
The spleen is necessary for PV development through amplification of the JAK2V617F erythroid compartment. A, Kinetics of spleen weight (left) and pictures of spleens 3 weeks after transplantation of WT and JAK2V617F BM cells (right). B, Kinetics of WT or JAK2V617F cells contribution to the LSK compartment. C, Kinetics of WT or JAK2V617F cells contribution to the megakaryocyte–erythroid progenitors (MEP) and the CD71+Ter119+ erythroid compartments in the spleen. D, Percentage of JAK2V617F LSK bar-coded clones shared between the irradiated leg, the nonirradiated leg, and the spleen. E, Venn diagrams of bar-coded CD71+Ter119+ erythroid (left top) and CD11b+Gr1+ myeloid clones (right top) in the nonirradiated leg and in the spleen and histograms showing the ratio of distribution of bar-coded erythroid (left bottom) and myeloid (right bottom) clones in the nonirradiated leg (NIR E, NIR M) and in the spleen (spleen E, spleen M; n = 3). F, Experimental design (top) and survival curve (bottom) of mice after splenectomy one week after transplantation of JAK2V617F or WT BM cells in a single irradiated leg. G, Kinetics of blood parameters in mice after splenectomy 1 week after transplantation of JAK2V617F or WT BM cells in a single irradiated leg. H, Kinetics of blood cell chimerism in mice after splenectomy one week after transplantation of JAK2V617F or WT BM cells in a single irradiated leg. I, JAK2V617F, WT and endogenous cells contributions to the irradiated and nonirradiated legs to the CD11b+Gr1+ myeloid and the CD71+Ter119+ erythroid compartments. J, JAK2V617F, WT and endogenous cells contributions to the irradiated and nonirradiated legs to the LT-HSC compartment 26 weeks after transplantation. K, Total hematopoietic cell number in the irradiated and nonirradiated legs 26 weeks after transplantation. Data are mean ±SEM from 8 to 12 mice. Significance was assessed using unpaired two-tailed t test and two-way ANOVA followed by post hoc analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; B and C, asterisks denote chimerism, and # denotes percentage of cells).