Abstract
Objectives
The objectives of this study were to investigate the utilisation patterns of pregabalin, to identify users potentially misusing pregabalin and to compare this group of patients to patients prescribed recommended doses of pregabalin concerning their personal characteristics and the coordination among their prescribers. Unintended coprescription of drugs with addictive potential might occur when care is insufficiently coordinated.
Design
Secondary data analysis of linked data from three regional sickness funds in Germany (AOK) for the years 2014–2016.
Setting
Ambulatory and hospital care sector in four German federal states.
Methods
On the basis of routine data, patients who received at least three prescriptions of pregabalin were identified and classified into patients prescribed pregabalin as recommended and those dispensed with a higher than recommended dose (>600 mg/day). Social network analysis was applied to identify prescription networks and to analyse cooperation among the prescribers. With descriptive statistics and univariate statistical tests, typical characteristics of the group of patients potentially misusing pregabalin were compared with the others.
Results
Among the 53 049 patients prescribed pregabalin, about 2% (877) were classified as potentially misusing pregabalin. The majority of this group was male and aged between 30 and 60 years. Of the patients misusing pregabalin, 365 (42%) had a diagnosed history of substance use disorders and 359 (41%) had been prescribed another drug with addictive potential (opioids) before. The prescribers of those patients potentially misusing pregabalin were more loosely connected within networks compared with prescribers of patients prescribed pregabalin as recommended.
Conclusion
This study found that patients could exceed recommended doses of pregabalin by getting prescriptions from multiple physicians. Specific patients were at increased risk of potentially misusing pregabalin, and these patients sought to obtain their prescriptions from physicians who were as loosely connected as possible. Coordination and sharing a relevant number of patients seem to be levers to avoid these problems of unintended coprescribing.
Keywords: health & safety, organisation of health services, quality in health care, therapeutics
Strengths and limitations of this study.
Routine data can serve as an objective measure to depict health service utilisation.
The applied methodology of social network analysis enables the exploration of cooperation among healthcare providers.
The study includes univariate statistical tests indicating differences between the two analysed groups of patients prescribed pregabalin but does not provide information about which analysed factors are most predictive.
The nature of routine data does not allow drawing conclusions about the reasons for high prescription rates and leads to incomplete information about prescriptions from the hospital sector or prescriptions not filled by the patients.
The analysed population is limited to people insured at the included three regional AOK sickness funds and might therefore differ slightly from the general population in Germany.
Introduction
The misuse or non-medical use of prescription drugs may lead to severe substance-related disorders and fatal health effects such as drug addiction, behavioural dependence or even death. The non-medical use of opioids is one of the leading public health issues in the USA1 and is characterised as an epidemic. Even though the prevalence is estimated to be lower in European countries, Novak et al2 reported past-year prevalence for non-medical drug use of up to 5% among five European Union member states. As many of these misused drugs have great addiction potential, patients may take advantage of coordination problems in healthcare systems, such as discontinuities or gaps in care.
One possible way for patients to misuse prescription drugs is to consume a higher than medically indicated dose.3 To this end, patients may seek to obtain prescriptions from multiple healthcare providers through so-called doctor shopping.3 Especially in fragmented healthcare systems, unknown and unintentional double prescribing might occur because patients can choose the physicians they consult, without the need for referral and information transfer among healthcare providers. This requires close cooperation and collaboration among providers when trying to prevent intentional misuse of prescription drugs, particularly when coordination gaps in healthcare are exploited by patients.
Pregabalin (Lyrica) is one example of such drugs, potentially misused by patients. It was introduced in 2004 and is approved for the treatment of neuropathic pain, general anxiety disorder and epilepsy in Europe. Pregabalin is a gamma-aminobutyric acid that reduces the excitability of neurons in the central nervous system and is structurally related to its predecessor gabapentin.4 Pregabalin binds to an auxiliary subunit of voltage-dependent calcium channels and thus reduces the release of several neurotransmitters such as glutamate, norepinephrine and the neuropeptide substance P.5 This may reduce neuronal excitability and thus seizures and neuropathic pain.6 Additionally, pregabalin may have a relaxing effect and can produce euphoria, which are both assumed to cause abuse and addictive potential.5
Since 2008, concerns have been raised about the abuse and addictive potential of pregabalin, particularly for patients with a history of drug addiction,4 7 8 and warning information was added to the German scientific information in 2011.9 Nevertheless, the number of patients prescribed pregabalin has still been growing in recent years.10 11 In Germany, an increase was observed from 2.2 million filled pregabalin prescriptions in 2011 to 3.9 million in 2018.12 13 Anecdotal evidence from Germany further suggests that there was also a rise in pregabalin abusers between 2008 and 2015.14 Despite these known issues, there exists no monitoring of prescription quantities of pregabalin in Germany.
Based on prescription data, studies have investigated patient factors that are associated with the risk of being dispensed with pregabalin at a higher than recommended dose.10 11 15 The authors interpreted this high dispensing of the drug as a sign of potential misuse of pregabalin. These studies showed that especially middle-aged men (between 18 and 45 years old), patients with a history of substance use disorders or drug abuse and patients with psychological comorbidities are at particularly high risk of misusing pregabalin. Driot et al15 found that, at a structural level, misuse of pregabalin was associated with multiple prescribers, which might point to the presence of doctor shopping.
Social network analysis (SNA) methods are commonly applied in the healthcare sector to identify network structures among healthcare providers and to investigate the effects of care cooperation among these informal, patient-sharing physician networks on healthcare provision.16 For instance, Barnett et al17 showed that, if physicians were sharing more patients in their empirical network, it was more likely that they were cooperating in real life. Making use of this idea, Ong et al18 used SNA to analyse networks of physicians prescribing interacting drugs to the same patients. They showed that a patient was more likely to be coprescribed with interacting drugs if his or her caring physicians shared fewer patients on average. In another study, Ong et al19 analysed multiple providers prescribing benzodiazepines and also showed that two physicians were at a greater risk of prescribing benzodiazepine with overlapping coverage if they shared fewer patients.
The German ambulatory care sector has no formal system to coordinate care among office-based physicians, and information about treatment and medication is not regularly transferred among healthcare providers. This loose organisation might facilitate the intentional misuse of prescription drugs for patients. The present study thus aimed to analyse pregabalin utilisation in four German states based on routinely collected health insurance data. It described the characteristics of patients who have been prescribed pregabalin and identified users potentially misusing this drug.1 This group was compared with the group of patients prescribed pregabalin as recommended in order to, first, examine the typical characteristics of patients misusing pregabalin and, second, identify the common factors and analyse the connectivity among the physicians prescribing pregabalin to patients who misuse the drug.
Methods
Data source and patient population
Three regionally organised statutory health insurances (AOK), covering four German states, Bavaria, Hesse, Thuringia and Saxony, provided sickness fund data for this study. In Germany, about 90% of the population is insured with a statutory health insurance and the AOK insures about 42% of the population in these regions (about 9.3 million persons). The insured population of the AOK differs only slightly from the general German population in terms of age and gender.20 The provided data set covered about 14% (about 1.25 million persons) of their insured population from the years 2013 to 2017.2 It included billed services and diagnoses from the ambulatory and hospital sector as well as prescription data and patient information, such as age and gender.
Patients were included in the analysis if they had received an initial prescription of pregabalin (Anatomical Therapeutic Chemical (ATC): N03AX16) between January 2014 and December 2016, ensuring that both a lead-up and a follow-up year for each patient was included in the data set. To be classified as an initial user, the patient should not have been prescribed pregabalin in the year prior to the initial prescription. Patients for whom only incomplete patient information was available, patients younger than 12 years of age and patients who died during the observation period of 1 year since their initial prescription were excluded from the analysis. Further, only patients with at least three filled prescriptions for pregabalin during the observation period of 1 year were considered to identify patients who used pregabalin regularly. Details about the identification of the patient population are depicted in figure 1.
Figure 1.
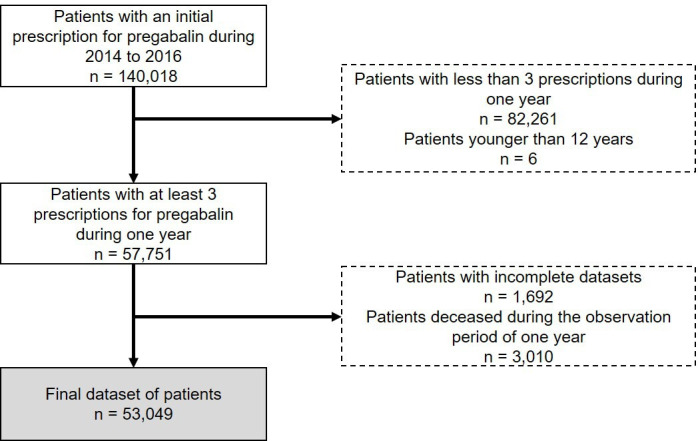
Identification of the analysed patient population in routine data.
Definition of potential misuse
The WHO defines psychoactive substance misuse as ‘Use of a substance for a purpose not consistent with legal medical guidelines […]’.21 The European public assessment report for pregabalin (Lyrica) recommends a maximum therapeutic dose of 600 mg/day (corresponding to two defined daily doses (DDD)).22 Therefore, to classify patients with prescriptions for pregabalin as patients prescribed pregabalin as recommended and those potentially misusing the drug, the average daily dose during 1 year was compared with this recommended maximum dose.10 11 15
The prescription data set listed all drugs that had been prescribed by any ambulatory physician and dispensed in a pharmacy. It provided the ATC classification, the prescription and dispensing date and the prescribed dose in terms of the DDD. On this basis, the sum of dispensed drugs per patient during the time span of a maximum of 12 months since initial dispensing was calculated, excluding the prescribed dose of the last dispense. Subsequently, the time span (in days) between the first and last dispensing was examined and the average amount of dispensed drug per day was calculated. If this average exceeded the maximum dose of 600 mg, the patient was classified as potentially misusing pregabalin with the hazard of behavioural dependence.
Patient characteristics and medical conditions
The patient characteristics and medical conditions that were used to describe the patient population and to compare the two groups included information about (a) patient characteristics, (b) prevalence of approved indications for pregabalin, (c) medical conditions that might increase the risk of misuse, and (d) prescriptions of drugs with potential for misuse as follows:
The data set comprised, among others, the age and gender as relevant patient characteristics, as studies have shown that especially men between 18 and 45 years old seem to be at higher risk of misusing pregabalin.10 11 15 As geographical variation among patients prescribed with pregabalin exists, for example, in Denmark,10 information about the district of patients’ place of residence was used to differentiate between patients living in urban areas and those living in rural ones.23
Diagnoses for the approved indications (neuropathic pain, general anxiety disorders and epilepsy) were identified using information about diagnoses from the hospital and ambulatory sector. To ensure that diagnoses were related to the pregabalin prescription, only diagnoses that had occurred no more than 3 months prior to the prescription were considered. The patterns of diagnosis codes are presented in the form of the International Classification of Diseases 10th Revision (ICD-10)24 and are summarised in table 1. The diagnoses were extracted from studies analysing indications associated with pregabalin prescriptions.11 15 25 26
Patients with a history of substance use disorders might be at higher risk of misusing pregabalin.11 Therefore, it was examined whether patients had been diagnosed with substance use disorders within two different quarters in hospital (‘main diagnosis’) or in the ambulatory sector (‘confirmed’) within 1 year prior to the initial pregabalin prescription. Additionally, it was examined whether patients had been prescribed a drug for the treatment of alcohol, tobacco or opioid addiction at least once in the year prior to the initial prescription (see table 1 for ICD-10 and ATC codes).
It was analysed whether patients had been prescribed opioids or psychostimulants (ATC N06B including centrally acting sympathomimetics, xanthine derivatives and other psychostimulants and nootropics such as meclofenoxate or pyritinol)27 in the year before the initial pregabalin prescription, because these drugs have a known potential for abuse and might therefore be more prevalent in the group of patients potentially misusing pregabalin. Since gabapentin as the predecessor of pregabalin is also under discussion because of its potential of abuse, it was also controlled for gabapentin prescriptions during the observation period (see table 1 for details).
Table 1.
Patterns of diagnoses (ICD-10) for relevant medical conditions and ATC for relevant prescriptions
| Indications/diagnoses/drugs | ICD-10/ATC codes |
| Approved indications* | |
| Epilepsy | G40; G41 |
| Generalised anxiety disorders | F41.1 |
| Neuropathic pain-related diagnoses | G35.9; G50.0; G50.1; G51.0; G53.0; G54.4; G54.6; G55.0; G55.1; G56.0; G56.2; G56.4; G56.9; G57.0; G57.1; G57.8; G57.9; G58.0; G58.7; G58.8; G62.9; G63.2; G82.1; G95.0; G95.2; G95.8; G97.9; I69.1; I69.3; M48.0; M50.1; M53.0; M53.1; M54.1; M54.3; M54.4; M79; M89.0; R52 |
| Additional neuropathic pain-related diagnoses (broad pattern) | B02; G13.0; G52.1; G56; G57; G58; G59; G60; G61; G62; G63; G99.0; M51.1; M54.2; T92.6; T93.6 |
| Substance use disorders† | F11–F19; T42; T43; Z71.4–5 |
| Addictive disorder drugs‡ | |
| Alcohol | N07BB |
| Tobacco | N07BA |
| Opioids | N07BC |
| Drugs with potential for abuse‡ | |
| Opioids | N02A |
| Psychostimulants | N06B |
| Benzodiazepines | N05B; N05C |
| Contemporaneous prescription of gabapentin§ | N03AX12 |
*Diagnoses during the same quarter as the initial prescription.
†At least one diagnosis in two different quarters during the year before the initial prescription.
‡At least one prescription during the year before the initial prescription.
§At least one prescription during the observation period of 1 year.
ATC, Anatomical Therapeutic Chemical; ICD-10, International Classification of Diseases 10th Revision.
Prescription networks and structural characteristics
To describe and analyse if and how patterns of utilisation differ between the two groups of patients prescribed pregabalin, a prescription network for each patient was identified. This approach allows an analysis of how strongly the prescribing physicians are connected in networks through shared patients. A large number of shared patients among the prescribers may indicate active cooperation including, for example, information transfer about dispensed drugs.17 In contrast, and assuming that doctor shopping is taking place, it was expected that the prescribers of patients who potentially misuse pregabalin are less connected to other prescribers. Thus, the hypothesis was that prescribers of patients who were identified as misusing pregabalin have fewer network contacts with other prescribers than those prescribers whose patients were prescribed with dosages as recommended.
The prescription networks were identified per patient for the observation period of 1 year since the initial prescription. They were first built as bipartite networks, in which a patient was connected to his or her prescribing physicians (see the prescription network in figure 2A). To analyse cooperation among these prescribing physicians and following the findings of patient-sharing network analyses, the group of patients in the bipartite networks was expanded to all patients seen by the prescribing physicians during the observation period (see the patient-sharing network in figure 2A). The resulting bipartite networks were subsequently summarised to unipartite networks, in which only the physicians were considered and connected through shared patients (see figure 2B). The care density, as a surrogate measure of care coordination among the physicians, was then calculated as the average number of shared patients between all possible pairs of providers in a patient’s prescription network.18 28
Figure 2.
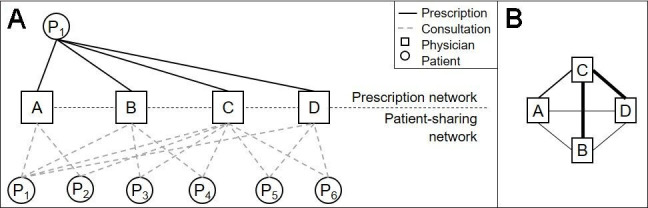
The bipartite prescription network of patient P1 and the resulting bipartite patient-sharing physician network of P1’s prescribers (A) and depiction of the resulting unipartite network (B). (A) The bipartite patient-sharing network of P1’s prescribers was calculated based on the extended patient population. (B) The thickness of tie represents the number of shared patients. The resulting care density is 1.83.
In the example in figure 2, four physicians filling at least one pregabalin prescription for patient P1 and the patients of the extended patient population who had consulted at least two of these four physicians are depicted. Comparing the patient populations of each physician, the number of patients shared between two physicians was examined and led to the unipartite network in figure 2B. The physicians in this network shared 1.83 patients on average, which was the care density of this network.
Other structural characteristics of the prescription networks included the number of filled prescriptions, the number of prescribers, the medical specialty of the first prescriber per patient and the proportion of specialised physicians among the prescribing physicians. Driot et al15 showed that the number of prescribers is associated with misuse of pregabalin. The medical specialty of initial prescriber and the specialty mix in the prescription networks may give insights into typical patterns of utilisation between the two groups of patients prescribed pregabalin.
Statistical methods
Mean values and SDs of the characteristics that were calculated as continuous variables are presented to describe the population of patients prescribed pregabalin. Characteristics that were collected as categorical variables are presented in terms of numbers and proportions. Univariate statistical tests were conducted to compare the group of patients prescribed pregabalin as recommended to the group of patients misusing pregabalin. To this end, the χ2 test was applied to categorical variables and, if a specification of a binary categorical variable contained fewer than five individuals, Fisher’s exact test was applied instead to that variable.29 Both tests were used to analyse whether the group proportions of a categorical variable were equal between the two groups.29 For continuous variables, the non-parametric Wilcoxon-Mann-Whitney U test was used to examine whether the values in one group were significantly greater (or smaller) than the values in the other group. This test does not require any assumptions about the distribution of the analysed variable, and the results can be considered conservative.29 In order to correct for multiple testing and the related increased risk of a type I error (false positives), a Bonferroni post hoc adjustment was conducted. This adjustment considers the number of statistical tests conducted to correct the resulting p values.30
Patient and public involvement
None.
Results
In total, 53 049 patients (ie, about 0.2% of the locality population insured with the AOK) received an initial pregabalin prescription between January 2014 and December 2016. During the 3 years, the absolute number of patients who were initially prescribed pregabalin and who received three or more prescriptions during 1 year increased from 17 003 in 2014 to 18 025 in 2016. In this group of patients, 877 (1.7%) were prescribed doses that on average exceeded the maximum therapeutic dose of 600 mg/day.
The descriptive statistics of all patients prescribed pregabalin and the results of the univariate statistical tests are summarised in table 2. The results indicate that the group of patients classified as patients prescribed pregabalin as recommended had similar characteristics to the total population for all presented values. However, the majority of characteristics differ systematically between the two groups built based on the amount of prescribed pregabalin.
Table 2.
Descriptive statistics of the data set and results of the univariate statistical analyses
| All patients prescribed pregabalin | Groups of patients with average doses | Unadjusted P value | Bonferroni adjusted P value | ||
| ≤600 mg/day | >600 mg/day | ||||
| n (%)/M (SD) | n (%)/M (SD) | n (%)/M (SD) | |||
| Patient characteristics | |||||
| Gender | |||||
| Male | 21 004 (39.6) | 20 468 (39.2) | 536 (61.1) | <0.001 | <0.001 |
| Female | 32 045 (60.4) | 31 704 (60.8) | 341 (38.9) | ||
| Age (years) | |||||
| 12–17 | 22 (0.0) | 22 (0.0) | 0 (0.0) | <0.001 | <0.001 |
| 18–29 | 867 (1.6) | 782 (1.5) | 85 (9.7) | ||
| 30–39 | 1996 (3.8) | 1808 (3.5) | 188 (21.4) | ||
| 40–49 | 4472 (8.4) | 4271 (8.2) | 201 (22.9) | ||
| 50–59 | 9434 (17.8) | 9252 (17.7) | 182 (20.8) | ||
| 60–69 | 9768 (18.4) | 9677 (18.5) | 91 (10.4) | ||
| ≥70 | 26 490 (49.9) | 26 360 (50.5) | 130 (14.8) | ||
| Place of residence | |||||
| Urban area | 23 862 (45.0) | 23 413 (44.9) | 449 (51.2) | <0.001 | 0.004 |
| Rural area | 29 119 (54.9) | 28 694 (55.0) | 425 (48.5) | ||
| Approved indications | |||||
| Epilepsy | 1968 (3.7) | 1882 (3.6) | 86 (9.8) | <0.001 | <0.001 |
| Generalised anxiety disorder | 3068 (5.8) | 2958 (5.7) | 110 (12.5) | <0.001 | <0.001 |
| Neuropathic pain | 39 829 (75.1) | 39 249 (75.2) | 580 (66.1) | <0.001 | <0.001 |
| Neuropathic pain (broad definition) | 42 120 (79.4) | 41 505 (79.6) | 615 (70.1) | <0.001 | <0.001 |
| Multiple | 3293 (6.2) | 3186 (6.1) | 107 (12.2) | <0.001 | <0.001 |
| None of the indications recorded in the records | 9283 (17.5) | 9098 (17.4) | 185 (21.1) | 0.005 | 0.135 |
| Medical preconditions with increased risk of abuse | |||||
| Substance use disorders | 6414 (12.1) | 6049 (11.6) | 365 (41.6) | <0.001 | <0.001 |
| Addictive disorder drug (alcohol) | 43 (0.1) | 41 (0.1) | 2 (0.2) | 0.345 | 1.000 |
| Addictive disorder drug (tobacco) | 2 (0.0) | 2 (0.0) | 0 (0.0) | 1.000 | 1.000 |
| Addictive disorder drug (opioids) | 258 (0.5) | 182 (0.3) | 76 (8.7) | <0.001 | <0.001 |
| Drugs with potential for abuse | |||||
| Benzodiazepine | 9665 (18.2) | 9367 (18.0) | 298 (34.0) | <0.001 | <0.001 |
| Opioids | 23 886 (45.0) | 23 527 (45.1) | 359 (40.9) | 0.015 | 0.386 |
| Psychostimulants | 288 (0.5) | 263 (0.5) | 25 (2.9) | <0.001 | <0.001 |
| Contemporaneous prescription of gabapentin | 2973 (5.6) | 2890 (5.5) | 83 (9.5) | <0.001 | <0.001 |
| Prescription networks and structural characteristics | |||||
| Number of prescriptions | 6.34 (3.28) | 6.23 (2.91) | 12.70 (10.17) | <0.001 | <0.001 |
| Number of prescribers (physicians) | 1.79 (1.03) | 1.77 (0.89) | 3.12 (3.91) | <0.001 | <0.001 |
| Number of prescribers (practices) | 1.59 (0.87) | 1.57 (0.73) | 2.86 (3.61) | <0.001 | <0.001 |
| Medical specialty of initial prescriber | |||||
| GP | 32 911 (62.0) | 32 344 (62.0) | 567 (64.7) | 0.010 | 0.256 |
| Anaesthesiology | 1935 (3.6) | 1908 (3.7) | 27 (3.1) | ||
| Orthopaedics | 1209 (2.3) | 1192 (2.3) | 17 (1.9) | ||
| Neuroscience | 4292 (8.1) | 4223 (8.1) | 69 (7.9) | ||
| Neurology | 5039 (9.5) | 4984 (9.6) | 55 (6.3) | ||
| Psychiatry and psychotherapy | 2341 (4.4) | 2289 (4.4) | 52 (5.9) | ||
| Other | 5322 (10.0) | 5232 (10.0) | 90 (10.3) | ||
| Proportion of specialists among prescribers | 0.31 (0.40) | 0.31 (0.40) | 0.31 (0.38) | 0.300 | 1.000 |
| Care density among physicians* | 47.97 (70.61) | 48.29 (70.67) | 33.23 (66.43) | <0.001 | <0.001 |
| Care density among practices* | 17.42 (35.77) | 17.54 (35.84) | 12.90 (32.76) | 0.149 | 1.000 |
| Maximal geographical distance (km) | 6.86 (26.63) | 6.71 (26.24) | 15.98 (43.27) | <0.001 | <0.001 |
*Care density was calculated as the average number of shared patients among all pairs of providers per patient and was calculated for patients with at least two prescribers (physicians/practices).
GP, general practitioner.
The results show that 32 045 (60%) patients prescribed pregabalin in the data set were female. The gender distribution was reversed for the group of patients misusing pregabalin: 536 patients (61%) were male and 341 (39%) female. Hence, the gender distribution differed significantly between the two groups of patients. Half of the patients were 70 years old or older when they received their initial pregabalin prescription, and there were only a few (22) patients who were between 11 and 18 years old. In contrast, in the group of patients misusing pregabalin, the age structure changed significantly, and most patients were between 30 and 60 years old.
Concerning the place of residence, it can be seen that, of all patients prescribed pregabalin, approximately 55% of patients (29 119) lived in rural areas and 45% (23 862) in urban areas. When focusing on patients misusing pregabalin, these values differed significantly in comparison to the other group of patients prescribed with less pregabalin: the majority of patients with high dosages of pregabalin lived in urban areas (51%; 449 patients), whereas the distribution of patients prescribed pregabalin as recommended was comparable to that of the total population.
Neuropathic pain was the most frequent indication that patients prescribed pregabalin were diagnosed within the same quarter as their initial prescription (39 829 patients (75%) and 42 120 patients (79%) for the broader definition). General anxiety disorder and epilepsy were prevalent in 3068 and 1968 patients, respectively. About 18% of patients (9283) had none of these diagnosed indications, and 6% of patients (3293) were diagnosed with several indications. In the group of patients potentially misusing pregabalin, the proportion of patients with no medical indication increased to 21% (185 patients). However, this result was not statistically significant after adjusting for multiple testing. Epilepsy and general anxiety disorder were more prevalent in the group of patients potentially misusing pregabalin compared with the other group, whereas the proportion of patients with neuropathic pain was slightly smaller. All these differences were found to be significant.
About 12% of patients (6414) had a history of substance use disorders, and the proportion of patients increased significantly to 42% (365) among patients potentially misusing pregabalin. Drugs for the treatment of addictive disorders were prescribed to only some patients in both groups, except drugs for the treatment of opioid addiction, which were prescribed to 76 patients (9%) with high dosages of pregabalin; this was significantly more than to the group of patients prescribed pregabalin as recommended (0.3%; 182 patients).
Overall, 9665 (18%) and 23 886 (45%) patients had been prescribed benzodiazepines or opioids, respectively, within the year prior to the initial pregabalin prescription. The proportion of patients with a prior prescription of benzodiazepine was significantly higher in the group of patients potentially misusing pregabalin (34%; 298), whereas 41% of these patients (359) had been prescribed with opioids during the year before.
Gabapentin was prescribed to 2973 (6%) of all patients prescribed pregabalin and to 83 (10%) of patients with high dosages of prescribed pregabalin.
Most of the patients received their initial pregabalin prescription from a general practitioner, followed by patients receiving their initial prescription from specialists in neuroscience or neurology. This characteristic varied only slightly and not significantly between the two groups of patients.
Patients prescribed pregabalin received on average six prescriptions from two different physicians over 1 year. In contrast, patients misusing pregabalin got on average about 13 prescriptions from three different physicians. Thus, their prescription networks were significantly larger than those of patients with recommended prescription doses were.
Lastly, physicians prescribing pregabalin to a patient with recommended dosages shared on average 48 patients. This value was significantly smaller (33 patients) among prescribers of patients who misuse pregabalin. The prescription networks of patients who were misusing pregabalin were thus less connected in terms of shared patients. In order to gain further insights into the prescription networks, the maximal geographical distance among the prescribers was calculated and compared between the two groups. It can be seen that, among the patients misusing pregabalin, the maximal distance was about 16 km on average, whereas the prescribers of the other group were less than half that distance away from each other.
Discussion
The presented study investigated the public health problem of the misuse of prescription drugs through coordination problems in healthcare systems, such as discontinuities or gaps in care. It included an extensive list of characteristics for analysing patients and their utilisation patterns of pregabalin. The list comprised both patient and structural characteristics of the prescribing physicians and was applied to patients from four German states. By taking advantage of routine data, all pregabalin prescriptions could be considered independently of the prescribing physicians. The data were used to identify a group of patients who were receiving a higher than medically recommended dose.
The investigated sample of patients prescribed pregabalin is comparable to patients presented in studies from other European countries regarding the age and gender structure of the patient population.10 11 26 The most prevalent medical indication in our study was neuropathic pain. This result is consistent with findings from other studies.11 15 31
The proportion of patients with high prescription volumes of pregabalin amounted to 877 in our sample (1.7%). Compared with the results of studies from Sweden with about 9%,11 Denmark with about 7%10 and France with almost 13%,15 this proportion is clearly smaller. Even though Novak et al2 showed that Germany has the lowest rates of drug misuse among the five analysed European countries, this difference might not only reflect a difference in prevalence but also be explained by slightly different approaches to identifying patients misusing pregabalin, for example, the German routine data do not include prescriptions filled by hospitals, or the fact that only patients with at least three prescriptions during 1 year were considered.
Evidence was found that particularly men aged 30–60 years and patients with a history of substance use disorders were over-represented in the group of patients misusing pregabalin. These results suggest that, among the patients prescribed pregabalin, there exists a group of patients who are at higher risk of misusing pregabalin and that physicians prescribing pregabalin should pay special attention to pre-existing medical conditions.
Compared with other studies, an unexpected result of this analysis is that compared with all patients prescribed pregabalin only relatively few patients potentially misusing pregabalin had a prior medication with opioids, as this was the case in other studies.4 32 At the same time, relatively high numbers of patients who had a prior medication with benzodiazepines were observed. Additionally, the proportion of patients with prior medication with drugs for the treatment of opioid addiction in the misusing group was high. One possible explanation for these results could be that pregabalin is sometimes used to relieve withdrawal symptoms from opioids or benzodiazepines, even though the drug is not approved for this application and the efficacy lacks evidence.33 Additionally, patients with neuropathic pain are also often treated with opioids or benzodiazepines and thus a consecutive prescription with pregabalin might be part of the treatment plan. However, this does not conclusively explain why there are significantly more patients with high amounts of pregabalin and a prior medication with benzodiazepines compared with patients prescribed pregabalin as recommended.
In addition to the presented patient characteristics that were associated with misuse of pregabalin, the study sheds light on the network structures of the prescribing ambulatory physicians. The results suggest that patients successfully attempted to get a higher than medically recommended dose of pregabalin. It has been shown that these patients had a greater number of prescribers (3.12 vs 1.77 physicians) and that their prescribing physicians were noticeably more loosely connected to other prescribers than those physicians whose patients were prescribed pregabalin in recommended doses (33 vs 48 shared patients). Additionally, the locations of the prescribers’ practices were further away from each other for patients misusing pregabalin compared with the other patients (16 vs 7 km). Both these results indicate that the patients are potentially seeking to receive prescriptions from physicians who are as unconnected with each other and geographically as far from each other as possible. Even though the reasons for the high prescription volumes in this group cannot be determined, these might be signs of existing doctor shopping when care coordination to control coprescriptions is not present.
To further analyse the group of patients potentially misusing pregabalin, a sensitivity analysis was conducted (see online supplemental material 2) in which the group of patients was differentiated between those receiving pregabalin from only one prescriber (practice) and those who were prescribed by multiple practices. The results indicate that, for example, the age structure of patients prescribed by only one provider shifted to higher ages (most of the patients were older than 50 years). Concerning the approved indications, it can be seen that there were more patients (24%; 71) in the group with only one prescriber who did not have any of the indications recorded in the data set compared with the group with multiple prescribers (20%; 114). At the same time, patients with only one prescriber were less likely to have received medications for addictive disorders or to have been prescribed other medications with addiction potential in the previous year. These results might indicate that being prescribed with a higher than recommended dose of pregabalin might not necessarily indicate doctor shopping or the lack of communication between healthcare providers but could also be medically explainable or caused by the data structure and a false classification (see also limitations).
bmjopen-2021-060104supp002.pdf (110KB, pdf)
Office-based physicians in Germany can be organised in group practices, and physicians from the same practice usually share a number of patients and might additionally represent each other in terms of filling prescriptions. Therefore, a large number of shared patients between physicians might primarily indicate that they belong to a common practice. In order to control for this issue, another sensitivity analysis was conducted using practices as the unit of prescribers instead of physicians and found comparable results (see table 2): patients prescribed pregabalin as recommended received their prescriptions from only 1.57 different practices on average, whereas patients misusing pregabalin had 2.86 different prescribing practices. In addition, in terms of care density, the practices in the prescription networks of patients potentially misusing pregabalin shared fewer patients than practices in the prescription networks of patients with medically recommended prescription doses (13 vs 18 shared patients). Even though this difference was not significant, these results support the conclusion that patients potentially misusing pregabalin seek to obtain prescriptions from loosely connected physicians and physicians who do not coordinate their care.
The application of SNA was used in the present analysis to examine a summary statistic of cooperation in order to compare the prescribers between the two groups of patients prescribed pregabalin. Future research could additionally visually compare prescription networks and use this methodology to identify clusters with a strikingly high prevalence of drug misuse.34
When interpreting the results, one has to take into account some important limitations. First, only pregabalin prescriptions and not gabapentin prescriptions were analysed, even though the abuse potential of gabapentin is also under discussion.4 However, as stated in the critical review report from the WHO, the risk of pregabalin misuse might be higher because of its stronger euphoric and relaxing effect.5 Second, the prescriptions included in the data set only covered prescriptions from office-based physicians, did not comprise medications that were provided during hospital stays and might thus have underestimated the amount of pregabalin consumed. Third, only one possible way of misusing pregabalin was considered in the study, that is, consuming a higher than recommended dosage, and did not consider other possibilities of misusing pregabalin, for example, the intake of drug combinations (eg, pregabalin and opioids). Fourth, the assumption that the patients with high prescription volumes of pregabalin are misusing the drug cannot conclusively be justified by the analysed data. For example, the data did not provide information about the compliance of patients, but only about the amount of drug dispensed. Thus, a conclusion about the final reason for high dispensed doses of pregabalin cannot definitely be drawn. Additionally, the sensitivity analysis differentiating the group of misusers into those with prescriptions from one or multiple providers points to the fact that this group might include some patients being falsely classified as ‘misusers’ and that there might exist other reasons for the high prescribed dosages. With the comparably low number of patients being classified as misusing pregabalin and an average dispensed dose of 905 mg per patient and per day within this group, the developed measure can be assumed as rather conservative that primarily discovers patients intentionally misusing pregabalin. However, a conclusive confirmation of this assumption can only be made by clinical studies that include patients and all their physicians.
Conclusion
To conclude, this study offers first insights into pregabalin utilisation and prescription patterns in Germany. Misuse of pregabalin is one example of patients’ intentional exploitation of coordination issues in ambulatory care. It sheds light on the evolving problems when care is not systematically coordinated and information about prescriptions is not exchanged. The study further shows how this problem might be minimised when physicians collaborate more closely, which is represented by a greater number of shared patients. However, absolute prevention of this problem will probably only be possible if information about medications is exchanged between all physicians as a standard and mandatory requirement. Last, the study discovered a group of patients who are potentially misusing this drug and shows that particularly prescribing physicians should be aware of this risk.
bmjopen-2021-060104supp001.pdf (17.5KB, pdf)
Supplementary Material
Acknowledgments
I thank all the cooperation partners for their support and collaboration within the project and for providing me with data. The project partners include members of the Department of General Medicine, Preventive and Rehabilitation Medicine, University of Marburg; AOK Health Insurance Hesse (SHI); AOK Health Insurance Bavaria (SHI); AOK PLUS, SHI in Thuringia and Saxony.
Footnotes
Contributors: RF designed and conducted the analysis, provided the results, wrote the manuscript, and is guarantor of the study.
Funding: This paper was written in the context of the WirtMed study, which was funded by the innovation fund programme of the German Federal Joint Committee (grant number: 01VSF17016).
Disclaimer: The funding source has no influence over the study or dissemination of the findings of the study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data processed in this study were provided by the three statutory health insurances (AOK PLUS, AOK Bavaria and AOK Hesse). However, restrictions apply to the availability of these data, which were used under licence for the current study (Innovation Fund project ‘WirtMed study’), and are not publicly available. Data are available from the authors upon reasonable request and with permission of the statutory health insurances and their supervisory authorities and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The present study was a secondary data analysis and therefore did not need an ethics approval. The data transmission from the participating statutory health insurances was organised via a trust centre, where the pseudonymised routine data were linked. To prevent reidentification, pseudonymised patient and physician data were pseudonymised again after the linkage and maintained in password-protected, encrypted containers. The following ministries in their role as supervisory authorities of the statutory health insurances approved the utilisation of the data: Bavarian State Ministry for Health and Care, Hessian Ministry for Social Affairs and Integration, and Saxon State Ministry for Social Affairs and Consumer Protection. The legal basis for the processing was given by Section 75 of Book X of the German Code of Social Law. By contract, inferences to individual patients are excluded and only aggregated results are presented.
References
- 1.National Institute On Drug Abuse . Misuse of prescription drugs research report, 2020. Available: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/what-scope-prescription-drug-misuse [Accessed 3 Dec 2020].
- 2.Novak SP, Håkansson A, Martinez-Raga J, et al. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016;16:1–12. 10.1186/s12888-016-0909-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur Addict Res 2012;18:228–45. 10.1159/000337028 [DOI] [PubMed] [Google Scholar]
- 4.Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs 2017;77:403–26. 10.1007/s40265-017-0700-x [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Critical review report: pregabalin. WHO expert committe drug depend forty-first meet (41st ECDD, 2018) 12–16 2018.
- 6.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007;73:137–50. 10.1016/j.eplepsyres.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 7.Schwan S, Sundström A, Stjernberg E, et al. A signal for an abuse liability for pregabalin – results from the Swedish spontaneous adverse drug reaction reporting system. Eur J Clin Pharmacol 2010;66:947–53. 10.1007/s00228-010-0853-y [DOI] [PubMed] [Google Scholar]
- 8.Schjerning O, Rosenzweig M, Pottegård A, et al. Abuse potential of pregabalin: a systematic review. CNS Drugs 2016;30:9–25. 10.1007/s40263-015-0303-6 [DOI] [PubMed] [Google Scholar]
- 9.Arzneimittelkommission der Ärzteschaft (akdä) . Abhängigkeitspotenzial von pregabalin (Lyrica). Dtsch Arztebl 2011:183. [Google Scholar]
- 10.Schjerning O, Pottegård A, Damkier P, et al. Use of pregabalin - a nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry 2016;49:155–61. 10.1055/s-0042-101868 [DOI] [PubMed] [Google Scholar]
- 11.Bodén R, Wettermark B, Brandt L, et al. Factors associated with pregabalin dispensing at higher than the approved maximum dose. Eur J Clin Pharmacol 2014;70:197–204. 10.1007/s00228-013-1594-5 [DOI] [PubMed] [Google Scholar]
- 12.Fricke U, Schwabe U. Neue Arzneimittel 2011. In: Arzneiverordnungs-report 2012. Heidelberg: Schwabe, Ulrich Paffrath, Dieter, 2012. [Google Scholar]
- 13.Knecht B, Lohmüller J, Telschow C. Ergänzende statistische Übersicht. In: Schwabe U, Paffrath D, Ludwig W-D, et al., eds. Arzneiverordnungs-Report 2019, 2019. [Google Scholar]
- 14.Zellner N, Eyer F, Zellner T. Alarmierender Pregabalin-Missbrauch: Prävalenz Im Münchener Raum, Konsummuster und Komplikationen. Dtsch Medizinische Wochenschrift 2017;142:e140–7. [DOI] [PubMed] [Google Scholar]
- 15.Driot D, Jouanjus E, Oustric S, et al. Patterns of gabapentin and pregabalin use and misuse: results of a population-based cohort study in France. Br J Clin Pharmacol 2019;85:1260–9. 10.1111/bcp.13892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuGoff EH, Fernandes-Taylor S, Weissman GE, et al. A scoping review of patient-sharing network studies using administrative data. Transl Behav Med 2018;8:598–625. 10.1093/tbm/ibx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett ML, Landon BE, O’Malley AJ, et al. Mapping physician networks with self-reported and administrative data. Health Serv Res 2011;46:1592–609. 10.1111/j.1475-6773.2011.01262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong M-S, Olson KL, Chadwick L, et al. The impact of provider networks on the co-prescriptions of interacting drugs: a claims-based analysis. Drug Saf 2017;40:263–72. 10.1007/s40264-016-0490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong M-S, Olson KL, Cami A, et al. Provider Patient-Sharing networks and multiple-provider prescribing of benzodiazepines. J Gen Intern Med 2016;31:164–71. 10.1007/s11606-015-3470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaunzeme J, Eberhard S, Geyer S. Wie repräsentativ sind GKV-Daten? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:447–54. 10.1007/s00103-012-1626-9 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Substance abuse Terminology & classification, 2020. Available: https://www.who.int/substance_abuse/terminology/abuse/en/ [Accessed 3 Dec 2020].
- 22.European Medicine Agency . European public assessment report (EPAR) for pregabalin Lyrica, 2004. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica [Accessed 2 Dec 2020].
- 23.Indikatoren und Karten zur Raum- und Stadtentwicklung (INKAR) . Zusammengefasste siedlungsstrukturelle Kreistypen. Bonn 2020.
- 24.World Health Organization (WHO) . Deutsches Institut für Medizinische Dokumentation und information (DIMDI) ICD-10-GM-2017, 2017. Available: https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2017/ [Accessed 3 Dec 2020].
- 25.Viniol A, Ploner T, Hickstein L, et al. Prescribing practice of pregabalin/gabapentin in pain therapy: an evaluation of German claim data. BMJ Open 2019;9:e021535. 10.1136/bmjopen-2018-021535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asomaning K, Abramsky S, Liu Q, et al. Pregabalin prescriptions in the United Kingdom: a drug utilisation study of the health improvement network (THIN) primary care database. Int J Clin Pract 2016;70:380–8. 10.1111/ijcp.12791 [DOI] [PubMed] [Google Scholar]
- 27.GKV-Arzneimittelindex im Wissenschaftlichen Institut der AOK . Anatomisch-therapeutisch-chemische Klassifikation mit Tagesdosen - Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland im Jahre 2017.
- 28.Pollack CE, Weissman GE, Lemke KW, et al. Patient sharing among physicians and costs of care: a network analytic approach to care coordination using claims data. J Gen Intern Med 2013;28:459–65. 10.1007/s11606-012-2104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heumann C, Schomaker M S. Hypothesis testing. In: Introduction to statistics and data analysis. 1st edn. Springer International Publishing, 2017: 209–42. [Google Scholar]
- 30.Bretz F, Hothorn T, Westfall P. General concepts and basic multiple comparison procedures. In: Multiple comparisons using R. 1st ed. CRC Press LLC, Boca Raton, 2010: 11–40. [Google Scholar]
- 31.Wettermark B, Brandt L, Kieler H, et al. Pregabalin is increasingly prescribed for neuropathic pain, generalised anxiety disorder and epilepsy but many patients discontinue treatment. Int J Clin Pract 2014;68:104–10. 10.1111/ijcp.12182 [DOI] [PubMed] [Google Scholar]
- 32.Grosshans M, Lemenager T, Vollmert C, et al. Pregabalin abuse among opiate addicted patients. Eur J Clin Pharmacol 2013;69:2021–5. 10.1007/s00228-013-1578-5 [DOI] [PubMed] [Google Scholar]
- 33.Freynhagen R, Backonja M, Schug S, et al. Pregabalin for the treatment of drug and alcohol withdrawal symptoms: a comprehensive review. CNS Drugs 2016;30:1191–200. 10.1007/s40263-016-0390-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Gallagher M, Loveday W, et al. Network analysis and visualisation of opioid prescribing data. IEEE J Biomed Health Inform 2020;24:1447–55. 10.1109/JBHI.2019.2939028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060104supp002.pdf (110KB, pdf)
bmjopen-2021-060104supp001.pdf (17.5KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data processed in this study were provided by the three statutory health insurances (AOK PLUS, AOK Bavaria and AOK Hesse). However, restrictions apply to the availability of these data, which were used under licence for the current study (Innovation Fund project ‘WirtMed study’), and are not publicly available. Data are available from the authors upon reasonable request and with permission of the statutory health insurances and their supervisory authorities and are not publicly available.


