Abstract
Background
Individuals residing in more socioeconomically disadvantaged neighborhoods experience greater uncertainty through insecurity of basic needs such as food, employment, and housing, compared with more advantaged neighborhoods. Although the neurobiology of uncertainty has been less frequently examined in relation to neighborhood disadvantage, there is evidence that neighborhood disadvantage is associated with widespread neural alterations.
Methods
Recently traumatically injured participants (n = 90) completed a picture anticipation task in the magnetic resonance imaging scanner, in which they viewed images presented in a temporally predictable or unpredictable manner. We investigated how neighborhood disadvantage (via area deprivation index [ADI]) was related to neural activation during anticipation and presentation of negative and neutral images after accounting for individual factors (i.e., age, gender, income, acute posttraumatic stress symptoms).
Results
There was a significant interaction during the anticipation period such that higher ADI rankings were related to greater activation of the right anterior cingulate cortex to predictable versus unpredictable neutral stimuli. Although no other robust interactions emerged related to ADI, we note several novel simple effects of ADI during anticipation and presentation periods in the hippocampus and prefrontal, cingulate, and occipital cortices.
Conclusions
Together, these results may represent an adaptive response to predictable and/or negative stimuli, stemming from chronic exposure to socioeconomic-based uncertainties. Although effects were modest, future work should continue to examine pretrauma context on posttrauma outcomes. To better understand trauma outcomes, it is imperative that researchers consider the broader context in which trauma survivors reside.
Keywords: ADI, Functional MRI, Neighborhood disadvantage, Socioeconomic position, Trauma, Uncertainty
The relationship between socioeconomic factors, including neighborhood disadvantage, and neurobiology has received considerable attention (1, 2, 3). Studies suggest that neighborhood disadvantage is associated with decreased volume of brain regions, widespread alteration of resting-state connectivity patterns, and changes to activation patterns during tasks (e.g., response inhibition, fear learning) (4, 5, 6, 7, 8). Although individuals living in more disadvantaged neighborhoods are faced with high levels of uncertainty (2,9) and greater exposure to trauma-related or injury-causing events (10), it remains unclear how neighborhood disadvantage may be impacting the neurobiological correlates of uncertainty, particularly in the aftermath of traumatic injury.
Environmental uncertainty is multifaceted: residents of more disadvantaged neighborhoods frequently experience insecurity of basic needs such as food, employment, and housing (11,12). Structural inequities and policies (e.g., underfunding of community resources) are underlying drivers of these insecurities, and their impact is far-reaching. For example, disadvantaged neighborhoods experience greater incidence of violence and are confronted with differential criminal justice practices (13,14). Similar to broader definitions of uncertainty, socioeconomic-based uncertainty, conceptualized as unpredictable yet frequent insecurities in essential needs and/or safety within the environment, activates the stress response in the brain and body (11,15).
The biological impact of socioeconomic-based uncertainty may transpire in adaptations to fear and anxiety neurocircuitry (16). Environmentally driven propensities toward hypervigilance, avoidance behaviors, and heightened reactivity can bias individuals to use ineffective coping strategies in the face of uncertainty, particularly during acutely stressful or traumatic experiences (17, 18, 19). These tendencies are well documented: living in disadvantaged neighborhoods is associated with hypervigilance and heightened reactivity to stimuli (20). The continuous deployment of cognitive resources and engagement in hypervigilant behaviors, which may be transiently adaptive, is further compounded by disproportionately elevated exposure to trauma in disadvantaged neighborhoods (10). A consequence of trauma exposure is posttraumatic stress disorder (PTSD), which is characterized by symptoms also linked to neighborhood disadvantage (e.g., hyperarousal) (16). Further, anticipation of uncertain events can elicit avoidance or defensive responses that, while useful in certain situations, can exacerbate symptoms of PTSD in objectively nonthreatening environments (21, 22, 23, 24). Clearly, the neurobiological and cognitive processes surrounding uncertainty are essential to understanding risk for mental health outcomes, particularly PTSD.
A wealth of research has shown that the anterior insula, cingulate cortex, dorsomedial and dorsolateral prefrontal cortex, amygdala, bed nucleus of the stria terminalis, and midbrain are consistently engaged during anticipation of negative or aversive events [reviewed in (25,26)]. The context (e.g., threat) in which uncertainty is tested differentially recruits specific regions. For example, the amygdala and anterior insula are most consistently activated during uncertain threat (26). The neurobiology of uncertainty has not been examined in relation to neighborhood disadvantage; however, there is evidence that neighborhood disadvantage is associated with widespread alterations to regions underlying affect. In children and adolescents, recent work has demonstrated extensive alterations to neural connectivity related to neighborhood deprivation at rest (6) as well as during response inhibition (7) and reward processing tasks (5). In young adults, Harnett et al. (4) demonstrated that negative life experiences, including neighborhood disadvantage, were associated with less amygdala and hippocampus activation in response to threat. Although uncertainty is alluded to in research on socioeconomic position and mental health outcomes (2,9), the influence of neighborhood disadvantage on the neural circuitry of uncertainty has remained uncharacterized.
This study evaluated a traumatically injured adult sample to investigate the intersection of neighborhood disadvantage, uncertainty neurobiology, and recent trauma exposure. This study builds off our previous work with this sample showing that neurocognitive (27) and resting-state aberrations (8) are related to neighborhood disadvantage. Specifically, greater neighborhood disadvantage was associated with greater connectivity between the amygdala and inferior parietal lobule and between the anterior insula and ventrolateral prefrontal cortex, suggesting an impact of neighborhood disadvantage on emotion regulation circuits (8). Even at rest, regions underlying uncertainty processing showed altered connectivity related to greater neighborhood disadvantage, although it is unclear if these alterations are relevant during tasks.
Therefore, this study sought to characterize neural activation during a task known to activate uncertainty neural circuitry. Given the relatively sparse work in this field, we used an exploratory whole-brain approach; however, we expected that activity in neural circuits known to modulate uncertainty processing (e.g., anterior insula, cingulate cortex, amygdala, dorsolateral prefrontal cortex) would be related to neighborhood disadvantage. Given the overlap in PTSD and uncertainty-related neural circuitry, this study aimed to evaluate the distinct effects of the environment on uncertainty beyond the effects of PTSD in a traumatically injured adult sample. We expected that neighborhood disadvantage would impact neural response to uncertain threat compared with uncertain neutral stimuli, after accounting for PTSD symptoms related to recent traumatic injury, individual socioeconomic position, and individual characteristics (i.e., gender, age, prior life trauma, income).
Methods and Materials
Participants
A total of 215 participants who recently experienced a traumatic injury were recruited from an Emergency Department at an urban level 1 trauma center as part of a longitudinal observational study investigating neurobiological and socioenvironmental predictors of PTSD [iSTAR study (8,27, 28, 29)]. Individuals were considered eligible if they were between the ages of 18 and 65 years old, were English speaking, had no contraindications for magnetic resonance imaging scanning, and had not sustained a head injury more severe than a mild traumatic brain injury. Participants provided written consent and were financially compensated for their time. All study procedures were approved by the local Institutional Review Board at the Medical College of Wisconsin.
Individual Measures
Sample characteristics can be found in Table 1. Individual demographics (gender, age, income, and acute PTSD symptoms) were self-reported at the first study visit. Annual household income was assessed on a semicontinuous scale (coded 1–11 in steps of $10,000; 1 = $0–$10,000/year, 11 = greater than $100,000).
Table 1.
Sample Characteristics (N = 90)
| Characteristics | Mean (SD) [Range] or % |
|---|---|
| Age, Years | 31.62 (9.46) [18–58] |
| Gender, Female/Male | 57%/42% |
| Race | |
| Asian | <5% |
| Black or African American | 53% |
| More than one race | 7% |
| White | 31% |
| Unknown | <5% |
| Income | |
| $0—$10,000 | 17% |
| $10,000–$20,000 | 18% |
| $20,000–$30,000 | 12% |
| $30,000–$40,000 | 7% |
| $40,000–$50,000 | 7% |
| $50,000–$60,000 | 6% |
| $60,000–$70,000 | 7% |
| $70,000–$80,000 | 5% |
| $80,000–$90,000 | <5% |
| $90,000–100,000 | 6% |
| Greater than $100,000 | 6% |
| Mechanism of Injury | |
| Motor vehicle crash | 65% |
| Stab | <5% |
| Pedestrian struck | <5% |
| Motorcycle crash | <5% |
| Domestic violence | <5% |
| Assault | 13% |
| Other | 10% |
| PCL-5 | 24.91 (17.43) [0–73] |
| ADI | 66.17 (23.92) [11–100] |
To ensure participant anonymity, small sample sizes in certain categories are reported as < 5%; thus, cumulative percentages may exceed 100%.
ADI, area deprivation index; PCL-5, PTSD Checklist for DSM-5.
Acute PTSD symptoms were measured using the PTSD Checklist Scale for DSM-5 (PCL-5) (30), which consists of 20 self-report items evaluating the severity of PTSD symptoms (31). A total PTSD symptom severity score was created by summing the scores of each item. This sample exhibited predominantly subthreshold PTSD symptoms (mean = 24.91, SD = 17.43) per the proposed clinical cutoff of 30 (32).
Neighborhood Socioeconomic Disadvantage
Neighborhood socioeconomic disadvantage was measured using the area deprivation index (ADI) (33). Participants’ home addresses were used to derive ADI rankings from a publicly available dataset through the University of Wisconsin School of Medicine and Public Health https://www.neighborhoodatlas.medicine.wisc.edu/ (downloaded February 2020, geocoding completed 3 months after all data were collected). Census block-group rankings were derived from data collected during the National 2014–2018 American Community Survey (33, 34, 35, 36). National ADI rankings are factor-based percentile scores representing 17 variables [see (36) for more information on ranking development] and range from 0 (most advantaged) to 100 (most disadvantaged) (36). The current sample lived in largely disadvantaged neighborhoods (mean ADI = 66, SD = 23).
Materials and Procedure
Picture Anticipation Task
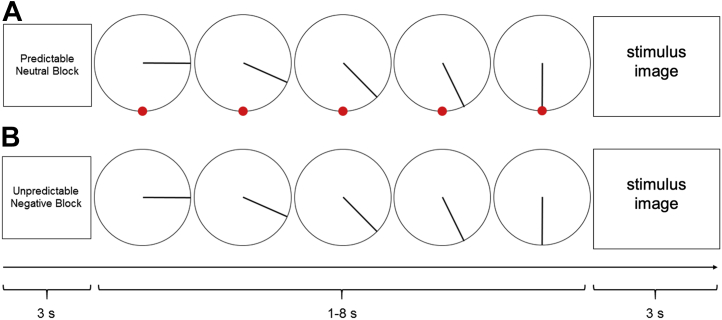
Participants completed four runs of the picture anticipation task, in which they viewed negative and neutral images presented in either a temporally predictable or unpredictable manner. In each run, two different condition blocks were presented from each of the four task conditions: predictable neutral, predictable negative, unpredictable neutral, and unpredictable negative. Blocks were presented pseudorandomly, such that there were never two blocks of the same condition within one run. There were eight total blocks, two of each condition, that lasted for approximately 91 seconds each.
In each block, subjects saw a 3-second start cue providing a description of the block condition. Each block contained 13 picture trials, with pictures displayed for 3 seconds. Prior to each picture was an anticipation period in which a ticking clock was displayed for 1 to 8 seconds (Figure 1). In the predictable blocks, the clock countdown accurately predicted the onset of the negative or neutral image (i.e., stimulus was displayed when the clock hand reached the red dot). In the unpredictable blocks, the movement of the clock hand was not related to the onset of the picture and no red dot was present. After 13 trials, a 3-second stop cue was presented at the end of the block signaling that it was finished. See the Supplement for additional details on the picture anticipation task, magnetic resonance imaging acquisition parameters, and preprocessing pipeline.
Figure 1.
An example of a trial of the picture anticipation task in which a predictable or unpredictable “clock” counts down to a neutral or negative image presentation. There were four conditions: unpredictable negative, unpredictable neutral, predictable negative, predictable neutral. Each block began with a 3-second cue. Each trial consisted of a 1–8-second countdown that preceded a 3-second image. (A) An example of a predictable neutral trial that had a predictable 5-second countdown, as depicted by the red dot. (B) An example of an unpredictable negative trial that had an unpredictable 5-second countdown, as depicted by no red dot.
Functional Magnetic Resonance Imaging Analysis
To model the anticipation period, blood oxygen level–dependent signal prior to image onset was modeled for each condition using AFNI’s duration modulation miniblock basis function, because the duration of the anticipation period was variable (1–8 seconds). To model transient response to the images, blood oxygen level–dependent signal at the onset of image presentation was modeled using a generalized linear model and a 14-second tent function with seven tents in AFNI: TENTzero(0,14,8).
Of the recruited participants, 165 participants had anatomical and task scans, and 99 had complete and usable scan data (n = 22 did not complete all runs of the task, n = 44 exceeded motion thresholds). Of the 99 with usable scan data, 6 were unable to be geocoded and 3 were missing other covariate data, yielding a final sample of n = 90.
Two voxelwise linear mixed effects models (3dLMEs) were run to investigate the relationship of ADI and whole-brain activation during 1) the anticipation period (ticking clock) and 2) presentation of task stimuli. The voxelwise statistical threshold was set at p < .001 and corrected for multiple comparisons across the whole brain at p < .05 using Monte Carlo simulations (k > 14) through AFNI’s 3dClustSim. In both models, valence (negative, neutral) and predictability (predictable, unpredictable) were within-subject effects. To remove any acute potential neurobiological consequences of a psychological posttraumatic response (37, 38, 39) and to isolate the effects of ADI, we included acute posttraumatic stress symptoms (PCL-5) as a covariate in the model. Additional covariates included age, gender, and income with ADI as the primary variable of interest. The following equation is an illustration of the full model:
Two additional sets of analyses were run and are reported in the Supplement. The first supplemental analysis demonstrates the effects of ADI without considering acute posttraumatic stress symptoms (i.e., PCL-5 was not included as a covariate). The second is a sensitivity analysis to determine whether the results were driven by individuals living in the most disadvantaged neighborhoods (8,40). We excluded participants with ADI rankings greater than 90 (n = 17) and reran the 3dLME analyses (n = 73).
Results
Anxiety Ratings
Six participants reported invalid or unexpected anxiety ratings (i.e., all the same number or in the exact opposite direction from expected); however, exclusion of these participants did not change the pattern of anxiety ratings across the task and are therefore retained in the reported results. Blocks with negative images elicited greater anxiety than blocks with neutral images (main effect of valence: F1,356 = 97.93, p < .001). Blocks with unpredictable timing did not elicit greater anxiety than blocks with predictable timing (main effect of predictability: F1,356 = 1.48, p = .19). There was no interaction of valence and predictability (F1,356 = 0.06, p = .80).
Picture Anticipation Task Effects: Anticipation Period
Results of the whole-brain voxelwise 3dLME examining activation during the anticipation period are presented in Table 2. After accounting for all covariates (i.e., ADI, gender, income, PCL-5, age), there was a main effect of predictability with greater activation in occipital and superior parietal regions for blocks anticipating predictable compared with unpredictable stimuli. There was a main effect of valence such that the right middle temporal gyrus showed greater activation in anticipation of neutral compared with negative stimuli. Finally, there was a significant predictability × valence interaction. In unpredictable blocks, the right posterior cingulate cortex showed greater activation during anticipation of neutral compared with negative images. In neutral blocks, the right posterior cingulate showed greater activation during unpredictable compared with predictable anticipation, and the left middle occipital gyrus showed greater activation to predictable compared with unpredictable anticipation.
Table 2.
Coordinates of Peak Whole-Brain Activation by Task Condition During Stimulus Anticipation Period (N = 90)
| Model Term | Condition | Brain Region | x | y | z | k | z |
|---|---|---|---|---|---|---|---|
| Predictability | P > U | Left middle occipital gyrus | −46 | −71 | 0 | 40 | 4.96 |
| Right superior occipital gyrus | 17 | −88 | 32 | 23 | 3.41 | ||
| Right superior parietal lobule | 35 | −53 | 60 | 15 | 4.77 | ||
| Valence | neu > neg | Right middle temporal gyrus | 45 | −67 | 0 | 22 | 3.86 |
| Valence × Predictability | Uneu > Uneg | Right middle cingulate cortex | 0 | −32 | 46 | 27 | 3.86 |
| Uneu > Pneu | Bilateral middle cingulate | 0 | −25 | 39 | 28 | 4.51 | |
| Pneu > Uneu | Left middle occipital gyrus | −46 | −71 | 0 | 22 | 5.16 | |
| Valence × Predictability | Pneu > Uneu and ADI | Right anterior cingulate cortex | 7 | 27 | 25 | 16 | 3.69 |
| Simple Effects | Uneu and ADI | Left hippocampus | −18 | −39 | −17 | 17 | −3.36 |
| Pneg and ADI | Left middle occipital gyrus | −39 | −81 | 0 | 41 | −5.16 | |
| Right inferior occipital gyrus | 31 | −92 | −7 | 22 | −4.37 | ||
| Right primary motor cortex | 45 | −1 | 32 | 15 | −5.05 | ||
| Left cerebellum | −25 | −78 | −45 | 14 | −4.46 | ||
| Pneu and ADI | Right hippocampus | 21 | −32 | −3 | 22 | 3.44 |
For results with ADI, gender, age, PTSD Checklist for DSM-5, and income were included as covariates in the model. Cluster thresholds: voxelwise: p < .001, clusterwise k > 14, p < .05. x, y, z, peak coordinates are in standard space (Montreal Neurological Institute152).
ADI, area deprivation index; k, cluster size; neg, negative; neu, neutral; P, predictable; U, unpredictable; z, z score.
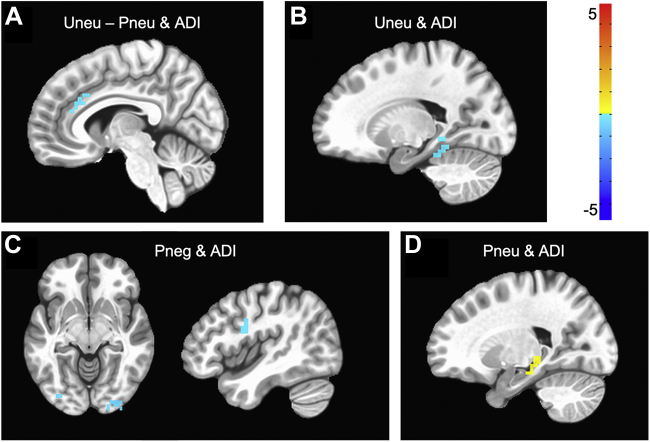
Anticipation period results of specific ADI effects of interest, after accounting for all other covariates, are depicted in Figure 2 and Table 2. There were no significant interactions of ADI with predictability or valence during anticipation. There was a significant three-way interaction of ADI × predictability × valence such that, in neutral blocks, greater neighborhood disadvantage was related to significant activation in the right anterior cingulate cortex (ACC) for predictable compared with unpredictable anticipation. For thoroughness, we note several simple effects of task conditions and ADI. In unpredictable neutral blocks, higher ADI rankings were related to less activation of the left hippocampus. In predictable negative blocks, higher ADI rankings were related to less activation of the bilateral occipital cortices, right primary motor cortex, and left cerebellum. Finally, in predictable neutral blocks, higher ADI rankings were related to greater activation of the right hippocampus.
Figure 2.
Significant clusters that emerged in relation to area deprivation index (ADI) and anticipation of given task conditions. Higher ADI rankings were related to (A) less activation in the anterior cingulate cortex to anticipation of unpredictable neutral (Uneu) stimuli as compared with predictable neutral (Pneu) stimuli, (B) less activation of the hippocampus in anticipation of unpredictable neutral, (C) less activation of visual cortices and the primary motor cortex in anticipation of predictable negative (Pneg), and (D) greater activation of the hippocampus in anticipation of predictable neutral stimuli. Cluster thresholds: voxelwise: p < .001, clusterwise k > 14, p < .05. N = 90.
Picture Anticipation Task Effects: Picture Presentation
Results of the whole-brain voxelwise 3dLME examining activation in response to stimulus presentation are detailed in Table 3. After accounting for all covariates, there was a significant main effect of valence such that the occipital and somatosensory cortices showed greater activation in response to negative versus neutral images. Further, in each of the unpredictable and predictable blocks, the occipital and somatosensory cortices showed greater activation in response to negative stimulus presentations compared with neutral presentations. However, there was no significant main effect of predictability.
Table 3.
Coordinates of Peak Whole-Brain Activation by Task Condition During Stimulus Presentation (N = 90)
| Model Term | Condition | Brain Region | x | y | z | k | z |
|---|---|---|---|---|---|---|---|
| Valence | neg > neu | Right occipital cortex | 35 | −78 | −14 | 1531 | 7.49 |
| Left occipital cortex | −35 | −81 | −17 | 1416 | 7.13 | ||
| Right primary motor cortex | 42 | 6 | 28 | 525 | 7.23 | ||
| Bilateral cerebellum | 0 | −57 | −38 | 210 | 6.80 | ||
| Right primary motor cortex | 42 | 3 | 35 | 129 | 6.79 | ||
| Left hippocampus | −25 | −29 | −3 | 61 | 6.87 | ||
| Left hippocampus | −21 | −1 | −14 | 26 | 4.04 | ||
| Left inferior frontal gyrus | −49 | 38 | 11 | 24 | 4.76 | ||
| Right thalamus | 21 | −32 | 4 | 18 | 4.65 | ||
| Right superior colliculi | 3 | −32 | −3 | 17 | 3.43 | ||
| Valence × Predictability | Uneg > Uneu | Right occipital cortex | 31 | −81 | −14 | 654 | 5.07 |
| Left occipital cortex | −39 | −71 | −17 | 374 | 4.83 | ||
| Right primary motor cortex | 42 | 6 | 32 | 149 | 5.34 | ||
| Left middle occipital gyrus | −28 | −74 | 25 | 69 | 4.24 | ||
| Left primary motor cortex | −42 | 3 | 28 | 34 | 5.54 | ||
| Right primary motor cortex | 38 | −1 | 49 | 14 | 5.17 | ||
| Pneg > Pneu | Right occipital cortex | 35 | −78 | −14 | 1013 | 5.65 | |
| Left occipital cortex | −35 | −81 | −17 | 934 | 5.57 | ||
| Left primary motor cortex | 42 | 6 | 28 | 209 | 5.78 | ||
| Bilateral cerebellum | 0 | −57 | −38 | 144 | 6.00 | ||
| Left primary motor cortex | −46 | 3 | 35 | 53 | 4.71 | ||
| Left hippocampus | −21 | −29 | −3 | 50 | 4.14 | ||
| Right inferior frontal gyrus | 49 | 38 | 11 | 34 | 4.60 | ||
| Left inferior frontal gyrus | −49 | 41 | 11 | 18 | 3.32 | ||
| Simple Effects | Uneg and ADI | Left inferior temporal gyrus | −49 | −43 | −17 | 24 | −4.09 |
| Uneu and ADI | Left hippocampus | −18 | −39 | −14 | 17 | −3.38 | |
| Pneg and ADI | Right anterior cingulate/lateral prefrontal | 42 | 34 | 11 | 81 | −5.26 | |
| Left lateral prefrontal cortex | −14 | 45 | −3 | 39 | −3.51 | ||
| Right primary motor cortex | 21 | 34 | 42 | 16 | −5.18 | ||
| Left temporal cortex | −46 | −39 | −17 | 14 | −3.51 | ||
| Pneu and ADI | Left temporal parietal junction | −56 | −64 | 21 | 76 | −3.31 | |
| Left posterior cingulate cortex | 0 | −43 | 32 | 58 | −3.53 | ||
| Left anterior prefrontal cortex | −21 | 66 | 4 | 30 | −3.41 | ||
| Left lingual gyrus | −14 | −60 | 11 | 18 | −3.48 |
For results with ADI, gender, age, PTSD Checklist for DSM-5, and income were included as covariates in the model. x, y, z, peak coordinates are in standard space (Montreal Neurological Institute152).
ADI, area deprivation index; k, cluster size; neg, negative; neu, neutral; P, predictable; U, unpredictable; z, z score.
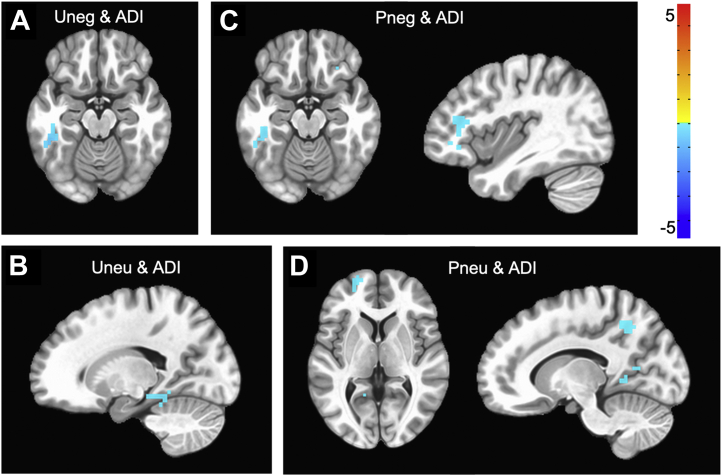
Stimulus presentation results of specific ADI effects of interest, after accounting for all other covariates, are depicted in Figure 3 and Table 3. There were no significant ADI × valence, ADI × predictability, or ADI × valence × predictability interactions. For thoroughness, we note that there were several simple effects of task conditions related to ADI. In response to unpredictable negative images, greater neighborhood disadvantage was related to less activation of the left temporal cortex. In response to unpredictable neutral images, higher ADI rankings were related to less activation of the left hippocampus. In response to predictable negative images, greater neighborhood disadvantage was related to less activation in the anterior cingulate, prefrontal, primary motor, and temporal cortices. Finally, in response to predictable neutral images, higher ADI rankings were related to less activation in the temporal, posterior cingulate, prefrontal, primary motor, and occipital cortices.
Figure 3.
Significant clusters that emerged in relation to area deprivation index (ADI) and presentation of stimuli for given task conditions. Greater neighborhood disadvantage was related to (A) less activation in inferior temporal gyrus to presentation of unpredictable negative (Uneg) stimuli, (B) less activation of the hippocampus to presentation of unpredictable neutral (Uneu) stimuli, (C) less activation of temporal and lateral prefrontal cortices to presentation of predictable negative (Pneg) stimuli, and (D) greater activation of prefrontal and posterior cingulate cortices to presentation of predictable neutral (Pneu) stimuli. Cluster thresholds: voxelwise: p < .001, clusterwise k > 14, p < .05. N = 90.
Discussion
In traumatically injured adults, we showed consistent recruitment of uncertainty processing regions (e.g., inferior frontal gyrus, hippocampus, visual cortices) during a picture anticipation task. We also demonstrated that neighborhood socioeconomic disadvantage is significantly associated with differences in the neural circuitry supporting affective processing. Specifically, we reported a significant interaction of pretrauma context during the anticipation period, but not in response to stimulus presentation, in the ACC. Although no other robust interactions emerged related to neighborhood socioeconomic disadvantage, we noted several novel simple effects. Socioeconomic-based uncertainty is more common for individuals residing in disadvantaged neighborhoods, and our results provide initial evidence that this exposure modulates neural activation. This is the first study to consider how the environment individuals are exposed to may affect the neurobiology of uncertainty in adults. Given the paucity of work at the intersection of neurobiology and neighborhood disadvantage in trauma populations, comparisons of our results with published literature are somewhat limited; nonetheless, we offer broad interpretations of our findings.
Overall Picture Anticipation Task Effects
After accounting for all covariates, the observed task activation during anticipation and stimulus presentation periods was consistent with the literature on trauma-exposed populations, with greater activation to negative than to neutral stimuli in the occipital and frontal cortices (41, 42, 43, 44). Some studies have found that hypervigilance to threat-related stimuli in PTSD can bias attention resources or require additional effort to disengage (42,45, 46, 47, 48, 49). However, others have found this same pattern for those who have experienced trauma broadly, regardless of PTSD diagnosis (41, 42, 43, 44). Thus, greater activation to negative stimuli in attention-related cortices, irrespective of PTSD severity, is consistent with previous reports of attentional biases toward negative and trauma-related stimuli in trauma survivors, particularly those without PTSD (43,44).
Effects of Picture Anticipation Task and Neighborhood Disadvantage
Although common posttrauma outcomes such as PTSD have frequently been shown to lead to hyperactivity in threat processing (47,48), this body of work has largely overlooked factors related to preexisting functioning and adaptations of threat-processing neural circuitry (4). The inclusion of pretrauma context (i.e., neighborhood disadvantage) and PTSD symptom severity as covariates in this study may help explain why we observed less activation in threat-related neural circuitry compared with the existing literature.
After accounting for individual characteristics (i.e., age, gender, income, and baseline PTSD symptoms), neighborhood disadvantage modulated activation during individual task conditions. Specifically, greater neighborhood disadvantage was related to less activation of the hippocampus in anticipation of unpredictable neutral stimuli, whereas anticipation of predictable neutral stimuli was related to greater hippocampal activation. The hippocampus is essential for regulating fear expression and consolidating fear memories (50). In this context, these results suggest that greater neighborhood disadvantage is related to less sensitivity to unpredictable stimuli, whereas those in more advantaged neighborhoods may respond to these stimuli as if they are more fearful. Along these lines, Harnett et al. (4), showed that negative life experiences lead to blunting of emotional function in brain (e.g., hypoactivity of hippocampus and amygdala during threat conditions) and behavior.
Greater neighborhood disadvantage was related to less activation of the ACC during anticipation of unpredictable versus predictable neutral stimuli. We note that this interaction emerged only during anticipation, and not during presentation, of neutral stimuli, suggesting a precise effect of pretrauma context on stimulus anticipation in the ACC. Further analysis of stimulus presentation effects showed that for predictable negative and neutral stimuli, higher ADI rankings were related to less activation of the ACC and posterior cingulate cortex, respectively. The ACC is critical for prediction of stimuli and execution of decision making in uncertainty (47,51). The posterior cingulate cortex is a critical node of the default mode network shown to be vulnerable to stress wherein reduced activity impairs top-down emotion regulation (52). This pattern of cingulate cortex activation is broadly consistent with previous work in trauma-exposed individuals. Less activation of the cingulate cortex in response to trauma-related stimuli is commonly reported in trauma populations with and without PTSD (45,53). Activation of the ACC has been shown to relate to PTSD symptom severity, although the direction of effect is widely debated in the literature and may depend on the comparison group and subregion of the ACC used in analysis (46,49,52).
Finally, in response to predictable neutral stimuli, higher ADI rankings were also associated with less activation of prefrontal, premotor, and occipital cortices. Higher ADI was also related to less activation of occipital cortices during the anticipation of and response to predictable negative stimuli. The direction of our findings is not unprecedented. In a study with veterans, less activation to conditioned unpredictable threat cues compared with predictable threat cues in trauma-exposed control subjects was reported in the amygdala, hippocampus, insula, and bilateral superior and middle temporal gyri, implying hypersensitivity to unpredictability (54). However, Dretsch et al. (54) did not account for prior life trauma, and thus inclusion of pretrauma context in this study may explain why our results showed less activation to unpredictable stimuli only in the occipital cortices and not in the amygdala, hippocampus, or insula. Moreover, Dretsch et al. (54) used a threatening auditory stimulus, whereas this study used visual stimuli and thus the superior and middle occipital gyri were recruited. Still, the majority of work in this realm has not considered pretrauma contexts, such as measures of neighborhood socioeconomic disadvantage, or even individual measures of socioeconomic circumstances (e.g., income).
Together, the results of this study may be understood as an adaptive response to unpredictable and/or negative stimuli, stemming from chronic exposure to socioeconomic-based uncertainties. We use adaptive here without a negative or positive connotation, rather to reflect an ecologically driven short-term change in neural circuitry suited to a given environment. The chronic unpredictability and stress of a disadvantaged context may sensitize emotion regulation and uncertainty processing circuitry, yielding less activation of these regions (4,55) as opposed to potentially harmful increases in activity and vigilance to uncertainty observed in anxiety disorders (56). We hypothesize that the chronic stress associated with greater neighborhood disadvantage may have altered reactivity to predictable stimuli given the endurance needed for maintaining and monitoring predictable and/or negative events in the environment. In other words, living with chronic stress makes living with unpredictability predictable, which impacts vigilance systems. We further suggest that uncertainty neural circuitry is not as engaged in laboratory tasks of unpredictability (51) given how frequent uncertainties occur in the lives of individuals residing in more disadvantaged environments (11,13, 14, 15). However, future investigations should specifically probe the relationship between uncertainty neural circuitry and socioeconomic-based uncertainties across different task contexts (e.g., reward, threat, safety).
Limitations
Several considerations temper the generalizability of our findings and the ability to infer how these alterations in neurobiology may be affecting the participants’ everyday lives. First, most inferences were drawn in response to observed simple effects because only one robust interaction with neighborhood socioeconomic disadvantage emerged from the full model. Given that the sample had just experienced a physical traumatic injury, although not worse than a mild traumatic brain injury, the recent exposure to trauma may impact threat-related processing beyond what can be captured by covarying for acute posttraumatic symptoms in the PCL-5. Unfortunately, there was excessive motion in the imaging data, which led to the exclusion of 44 participants. The nature of the task (i.e., presentation of negative stimuli and mixed design) resulted in a subset of participants (n = 22) who were either unable or uncomfortable with completing all necessary trials (all four runs). The exclusion of these participants was also unfortunate because it greatly limited our ability to examine other factors that may interact with socioeconomic-based uncertainty. For example, negative life experiences have been shown to help explain racial differences in threat processing (4).
Racial identity and neighborhood disadvantage are independent, although not mutually exclusive (57); both constructs underscore means by which chronic environmental stress can impact neurobiology and trauma outcomes. In the United States, members of minoritized racial and ethnic groups are disproportionately exposed to neighborhood disadvantage. Racialized socioeconomic inequities are considered drivers of racial and ethnic health disparities, and alterations to neurobiology may be just one mechanism linking these inequities to health outcomes. Different aspects of identity, such as socioeconomic circumstances, race, ethnicity, and gender identity, form complex connections that shape how an individual responds in different contexts (58,59). We call for future neuroscience research, in trauma populations and otherwise, to consider using an intersectional approach to examine the independent effects and interactions between sociodemographic variables (60). Finally, this study did not evaluate the duration of exposure to current socioeconomic circumstances, including neighborhood disadvantage and income. Childhood socioeconomic position impacts neurodevelopment and influences adulthood socioeconomic position. Therefore, future work should consider evaluating how stability of income and ADI interacts with neural processing of uncertainty.
Conclusions
The effects of neighborhood and contextual factors on the brain and psychophysiological outcomes are becoming apparent with a renewed resurgence of research in the field. The majority of findings show a detrimental effect of greater neighborhood disadvantage on brain health. For example, previous work has shown that neighborhood disadvantage is related to decreased hippocampal volume in healthy (61) and trauma-exposed adults (27), as well as decreased white matter integrity of cingulum, uncinate, and stria terminalis/fornix pathways (40). Here, we present a more nuanced picture of the effects of neighborhood disadvantage on neural circuitry. Although effects were modest, results suggest that residing in a disadvantaged neighborhood was associated with modifications in regions subserving uncertainty processing, making it clear that future work should continue to examine pretrauma context on posttrauma outcomes. Although these modifications may be adaptive when faced with chronic uncertainties in daily life, they may also represent a predisposition to poor outcomes if trauma wreaks havoc on the brain. Poverty-related stressors have been associated with many outcomes, including anxiety, depression, social issues, cognition, and pain (2). The results of this study add to a growing body of work demonstrating alterations of neural circuitry in traumatically injured adults related to neighborhood disadvantage. To better understand trauma outcomes, it is imperative that researchers, policy makers, and communities characterize and address the drivers of systemic inequities contributing to health disparities.
Acknowledgments and Disclosures
The work within this article was supported by the National Institute of Mental Health (Grant No. R01-M1H106574 [principal investigator, CLL]) and a Medical College of Wisconsin CTSI Pilot Award (principal investigator: TAd-C). EKW was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (Grant No. 2TL1TR001437).
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
We thank the iSTAR research team and all participants that took part in the study—without their commitment, this work would not have been possible.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.02.006.
Supplementary Material
References
- 1.Glymour M.M., Mujahid M., Wu Q., White K., Tchetgen Tchetgen E.J. Neighborhood disadvantage and self-assessed health, disability, and depressive symptoms: Longitudinal results from the health and retirement study. Ann Epidemiol. 2010;20:856–861. doi: 10.1016/j.annepidem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santiago C.D., Wadsworth M.E., Stump J. Socioeconomic status, neighborhood disadvantage, and poverty-related stress: Prospective effects on psychological syndromes among diverse low-income families. J Econ Psychol. 2011;32:218–230. [Google Scholar]
- 3.Wadsworth M.E., Evans G.W., Grant K., Carter J.S., Duffy S. In: Developmental Psychopathology. Cicchetti D., editor. John Wiley & Sons, Inc.; Hoboken: 2016. Poverty and the development of psychopathology; pp. 1–44. [Google Scholar]
- 4.Harnett N.G., Wheelock M.D., Wood K.H., Goodman A.M., Mrug S., Elliott M.N., et al. Negative life experiences contribute to racial differences in the neural response to threat. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins T.S., Campbell E.M., Hogeveen J. Neighborhood deprivation shapes motivational-neurocircuit recruitment in children. Psychol Sci. 2020;31:881–889. doi: 10.1177/0956797620929299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakesh D., Seguin C., Zalesky A., Cropley V., Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: The moderating role of positive family and school environments. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson R.C., Burt S.A., Waller R., Jonides J., Miller A.L., Gearhardt A.N., et al. Neighborhood poverty predicts altered neural and behavioral response inhibition. NeuroImage. 2020;209 doi: 10.1016/j.neuroimage.2020.116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb E.K., Weis C.N., Huggins A.A., Fitzgerald J.M., Bennett K.P., Bird C.M., et al. Neural impact of neighborhood socioeconomic disadvantage in traumatically injured adults. Neurobiol Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang T., Yang X.Y., Yu L., Cottrell R.R., Jiang S. Individual and regional association between socioeconomic status and uncertainty stress, and life stress: A representative nationwide study of China. Int J Equity Health. 2017;16:118. doi: 10.1186/s12939-017-0618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook A., Harris R., Brown H.E., Bedrick E. Geospatial characteristics of non-motor vehicle and assault-related trauma events in greater Phoenix, Arizona. Inj Epidemiol. 2020;7:34. doi: 10.1186/s40621-020-00258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laraia B.A., Leak T.M., Tester J.M., Leung C.W. Biobehavioral factors that shape nutrition in low-income populations: A narrative review. Am J Prev Med. 2017;52:S118–S126. doi: 10.1016/j.amepre.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opačić A. In: Practicing Social Work in Deprived Communities. Opačić A., editor. Springer International Publishing; Cham, Germany: 2021. Effects of living in disadvantaged neighbourhoods on personal well-being; pp. 37–67. [Google Scholar]
- 13.Baglivio M.T., Wolff K.T., Epps N., Nelson R. Predicting adverse childhood experiences: The importance of neighborhood context in youth trauma among delinquent youth. Crime Delinquency. 2017;63:166–188. [Google Scholar]
- 14.Maguire-Jack K., Font S.A. Community and individual risk factors for physical child abuse and child neglect: Variations by poverty status. Child Maltreat. 2017;22:215–226. doi: 10.1177/1077559517711806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters A., McEwen B.S., Friston K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol. 2017;156:164–188. doi: 10.1016/j.pneurobio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Grupe D.W., Nitschke J.B. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DaViera A.L., Roy A.L., Uriostegui M., Fiesta D. Safe spaces embedded in dangerous contexts: How Chicago youth navigate daily life and demonstrate resilience in high-crime neighborhoods. Am J Community Psychol. 2020;66:65–80. doi: 10.1002/ajcp.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santiago C.D., Etter E.M., Wadsworth M.E., Raviv T. Predictors of responses to stress among families coping with poverty-related stress. Anxiety Stress Coping. 2012;25:239–258. doi: 10.1080/10615806.2011.583347. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth M.E. Development of maladaptive coping: A functional adaptation to chronic, uncontrollable stress. Child Dev Perspect. 2015;9:96–100. doi: 10.1111/cdep.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith N.A., Voisin D.R., Yang J.P., Tung E.L. Keeping your guard up: Hypervigilance among urban residents affected by community and police violence. Health Aff (Millwood) 2019;38:1662–1669. doi: 10.1377/hlthaff.2019.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fetzner M.G., Horswill S.C., Boelen P.A., Carleton R.N. Intolerance of uncertainty and PTSD symptoms: Exploring the construct relationship in a community sample with a heterogeneous trauma history. Cogn Ther Res. 2013;37:725–734. [Google Scholar]
- 22.McNaughton N., Gray J.A. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- 23.McTeague L.M., Lang P.J. The anxiety spectrum and the reflex physiology of defense: From circumscribed fear to broad distress. Depress Anxiety. 2012;29:264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen J.B., Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 25.Grupe D.W., Oathes D.J., Nitschke J.B. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morriss J., Gell M., van Reekum C.M. The uncertain brain: A co-ordinate based meta-analysis of the neural signatures supporting uncertainty during different contexts. Neurosci Biobehav Rev. 2019;96:241–249. doi: 10.1016/j.neubiorev.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Webb E.K., Weis C.N., Huggins A.A., Parisi E.A., Bennett K.P., Miskovich T., et al. Neighborhood disadvantage is associated with stable deficits in neurocognitive functioning in traumatically-injured adults. Health Place. 2021;67 doi: 10.1016/j.healthplace.2020.102493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird C.M., Webb E.K., Schramm A.T., Torres L., Larson C., deRoon-Cassini T.A. Racial discrimination is associated with acute posttraumatic stress symptoms and predicts future posttraumatic stress disorder symptom severity in trauma-exposed black adults in the United States. J Trauma Stress. 2021;34:995–1004. doi: 10.1002/jts.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weis C.N., Webb E.K., Stevens S.K., Larson C.L., deRoon-Cassini T.A. Scoring the Life Events Checklist: Comparison of three scoring methods. Psychol Trauma. 2022;14:714–720. doi: 10.1037/tra0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. J Trauma Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . American Psychiatric Publishing; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Health Disorders: DSM-5. [Google Scholar]
- 32.Geier T.J., Hunt J.C., Nelson L.D., Brasel K.J., deRoon-Cassini T.A. Detecting PTSD in a traumatically injured population: The diagnostic utility of the PTSD Checklist for DSM-5. Depress Anxiety. 2019;36:170–178. doi: 10.1002/da.22873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible — The neighborhood atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J., Kind A.J.H., Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33:493–501. doi: 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kind A.J., Jencks S., Brock J., Yu M., Bartels C., Ehlenbach W., et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161:765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh G.K. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harnett N.G., van Rooij S.J.H., Ely T.D., Lebois L.A.M., Murty V.P., Jovanovic T., et al. Prognostic neuroimaging biomarkers of trauma-related psychopathology: Resting-state fMRI shortly after trauma predicts future PTSD and depression symptoms in the AURORA study. Neuropsychopharmacology. 2021;46:1263–1271. doi: 10.1038/s41386-020-00946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roeckner A.R., Oliver K.I., Lebois L.A.M., van Rooij S.J.H., Stevens J.S. Neural contributors to trauma resilience: A review of longitudinal neuroimaging studies. Transl Psychiatry. 2021;11:508. doi: 10.1038/s41398-021-01633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Rooij S.J.H., Stevens J.S., Ely T.D., Hinrichs R., Michopoulos V., Winters S.J., et al. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol Psychiatry. 2018;84:106–115. doi: 10.1016/j.biopsych.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell K.L., Purcell J.B., Harnett N.G., Goodman A.M., Mrug S., Schuster M.A., et al. White matter microstructure in the young adult brain varies with neighborhood disadvantage in adolescence. Neuroscience. 2021;466:162–172. doi: 10.1016/j.neuroscience.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schäfer J., Zvielli A., Höfler M., Wittchen H.U., Bernstein A. Trauma, attentional dysregulation, and the development of posttraumatic stress: An investigation of risk pathways. Behav Res Ther. 2018;102:60–66. doi: 10.1016/j.brat.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Blair K.S., Vythilingam M., Crowe S.L., McCaffrey D.E., Ng P., Wu C.C., et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2013;43:85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimble M.O., Fleming K., Bandy C., Zambetti A. Attention to novel and target stimuli in trauma survivors. Psychiatry Res. 2010;178:501–506. doi: 10.1016/j.psychres.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsen A.S., Blix I., Leknes S., Ekeberg Ø., Skogstad L., Endestad T., et al. Brain activity in response to trauma-specific, negative, and neutral stimuli. A fMRI study of recent road traffic accident survivors. Front Psychol. 2016;7:1173. doi: 10.3389/fpsyg.2016.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fani N., King T.Z., Clendinen C., Hardy R.A., Surapaneni S., Blair J.R., et al. Attentional control abnormalities in posttraumatic stress disorder: Functional, behavioral, and structural correlates. J Affect Disord. 2019;253:343–351. doi: 10.1016/j.jad.2019.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzgerald J.M., DiGangi J.A., Phan K.L. Functional neuroanatomy of emotion and its regulation in PTSD. Harv Rev Psychiatry. 2018;26:116–128. doi: 10.1097/HRP.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes J.P., VanElzakker M.B., Shin L.M. Emotion and cognition interactions in PTSD: A review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White S.F., Costanzo M.E., Blair J.R., Roy M.J. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage Clin. 2015;7:19–27. doi: 10.1016/j.nicl.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin J., Maren S. Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Sci Rep. 2015;5:8388. doi: 10.1038/srep08388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holz N.E., Tost H., Meyer-Lindenberg A. Resilience and the brain: A key role for regulatory circuits linked to social stress and support. Mol Psychiatry. 2020;25:379–396. doi: 10.1038/s41380-019-0551-9. [DOI] [PubMed] [Google Scholar]
- 52.Hinojosa C.A., Kaur N., VanElzakker M.B., Shin L.M. Cingulate subregions in posttraumatic stress disorder, chronic stress, and treatment. Handb Clin Neurol. 2019;166:355–370. doi: 10.1016/B978-0-444-64196-0.00020-0. [DOI] [PubMed] [Google Scholar]
- 53.Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dretsch M.N., Wood K.H., Daniel T.A., Katz J.S., Deshpande G., Goodman A.M., et al. Exploring the neurocircuitry underpinning predictability of threat in soldiers with PTSD compared to deployment exposed controls. Open Neuroimag J. 2016;10:111–124. doi: 10.2174/1874440001610010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno-López L., Ioannidis K., Askelund A.D., Smith A.J., Schueler K., van Harmelen A.L. The resilient emotional brain: A scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:392–402. doi: 10.1016/j.bpsc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Hiser J., Schneider B., Koenigs M. Uncertainty potentiates neural and cardiac responses to visual stimuli in anxiety disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:725–734. doi: 10.1016/j.bpsc.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams D.R., Lawrence J.A., Davis B.A. Racism and health: Evidence and needed research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson P., Williams D. Jossey-Bass Publishers; San Francisco: 2006. The Intersection of Race, Gender, and SES: Health Paradoxes; pp. 131–162. [Google Scholar]
- 59.Potter L., Zawadzki M.J., Eccleston C.P., Cook J.E., Snipes S.A., Sliwinski M.J., Smyth J.M. The intersections of race, gender, age, and socioeconomic status: Implications for reporting discrimination and attributions to discrimination. Stigma Health. 2019;4:264–281. doi: 10.1037/sah0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seaton E.K., Caldwell C.H., Sellers R.M., Jackson J.S. An intersectional approach for understanding perceived discrimination and psychological well-being among African American and Caribbean Black youth. Dev Psychol. 2010;46:1372–1379. doi: 10.1037/a0019869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunt J.F.V., Buckingham W., Kim A.J., Oh J., Vogt N.M., Jonaitis E.M., et al. Association of neighborhood-level disadvantage with cerebral and hippocampal volume. JAMA Neurol. 2020;77:451–460. doi: 10.1001/jamaneurol.2019.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.