Abstract
Aim
Sunitinib is an oral tyrosine kinase inhibitor approved for the treatment of renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST). Because of the large interpatient pharmacokinetic variability and established exposure‐response and exposure‐toxicity relationships in clinical trial patients, therapeutic drug monitoring (TDM) seems promising for optimizing sunitinib exposure. We aimed to investigate the relationship between sunitinib exposure and treatment outcome in a real‐world patient cohort.
Methods
We performed a retrospective observational cohort study in 53 patients with metastatic RCC and 18 patients with metastatic GIST treated with sunitinib and receiving TDM‐guided dosing. Time on treatment – as a surrogate for progression‐free survival – in patients who achieved adequate sunitinib exposure was compared with patients who did not. Additionaly, the median sunitinib exposure was compared in patients with or without sunitinib‐induced toxicity leading to dose reduction.
Results
The median time on treatment in patients with RCC who achieved adequate sunitinib exposure (n = 39) was 32 weeks, compared to 15 weeks in patients who did not achieve adequate sunitinib exposure (n = 12) (P = 0.244). In 29 patients (41%) with toxicity leading to dose reduction, sunitinib sum plasma trough concentration (C trough) until dose reduction was significantly higher compared to patients without toxicity leading to dose reduction (median 60 ng/mL vs 44 ng/mL; P < 0.001) and reduced to comparable levels after dose reduction (44 ng/mL; P = 0.488).
Conclusion
In our real‐world patient cohort, patients with sunitinib‐induced toxicity requiring dose reduction had significantly higher sunitinib exposure compared to patients without toxicity. The threshold for toxicity, however, was lower compared to that previously described in clinical trials.
Keywords: pharmacodynamics, pharmacokinetics, sunitinib, therapeutic drug monitoring
What is already known about this subject
Because of large interpatient pharmacokinetic variability and an established exposure‐outcome relationship, therapeutic drug monitoring (TDM) for sunitinib seems promising.
In clinical trials, therapeutic windows for sunitinib exposure for continuous dosing and for intermittent dosing have been established.
Clinical trial patients differ substantially from real‐world patients.
What this study adds
Sunitinib exposure is significantly higher in patients with sunitinib‐induced toxicity leading to dose reduction compared to patients without toxicity.
The threshold for toxicity is lower in real‐world patients compared to patients in clinical trials.
With TDM‐guided dose adjustments, the number of patients reaching adequate sunitinib exposure increased to 92%. Overall an adequate sunitinib was reached in 53% of patients.
1. INTRODUCTION
Sunitinib is an oral tyrosine kinase inhibitor (TKI) that is currently registered for the treatment of advanced or metastatic renal cell carcinoma (RCC), gastrointestinal stromal tumour (GIST) and neuroendocrine tumour (NET). 1 , 2 , 3 Sunitinib is an inhibitor of vascular endothelial growth factor receptors 1, 2 and 3 (VEGFR1‐3), platelet‐derived growth factor receptors α and β (PDGFRα‐β), fetal liver tyrosine kinase receptor 3 (FLT3), the receptor for stem cell factor (KIT), colony‐stimulating factor receptor (CSF‐1R) and glial cell‐line derived neurotrophic factor receptor (RET), 4 , 5 , 6 which results in inhibition of tumour growth, neoangiogenesis and metastatic progression. Both intermittent and continuous dosing schedules are used. Patients with RCC are generally treated with a dose of 50 mg once a day (OD), either 4 weeks on, 2 weeks off (4/2) or 2 weeks on, 1 week off (2/1), although some patients are treated with a continuous dosing schedule of 37.5 mg OD. 7 , 8 Patients with GIST and NET are more often treated with the continuous dosing schedule. 3 , 9
Many TKIs are administered orally at a flat‐fixed dose and large interpatient pharmacokinetic (PK) variability is observed. Therapeutic drug monitoring (TDM) comprises the measurement and interpretation of drug concentrations in biological fluids to assist in rational drug dosage individualisation. Because of the large interpatient PK variability and the fact that relationships between exposure and both efficacy and toxicity have been established for several TKIs, TDM‐guided dosing seems promising. 10 , 11 , 12 , 13 This applies in particular for imatinib, sunitinib and pazopanib, in which therapeutic windows have been defined and the feasibility of TDM to reach drug levels within these therapeutic windows has been shown. 14 , 15 , 16 , 17 , 18 Therefore, TDM‐guided dosing is considered viable for these three drugs and is currently being implemented as a standard of care in the Netherlands. 12 , 19
In the metabolism of sunitinib, an equipotent active metabolite is produced (desethylsunitinib, SU012662) at concentration levels that substantially contribute to the total exposure at steady state. 4 , 20 , 21 Sunitinib sum plasma trough concentration (C trough) represents the sum of both sunitinib and the metabolite SU012662 concentrations. A therapeutic window for sunitinib sum C trough of 50‐87.5 ng/mL for intermittent dosing and 37.5‐75 ng/mL for continuous dosing has been established. 12 , 15 , 20 These therapeutic windows were mainly based on retrospective pooled data analyses of patients who participated in clinical phase I‐III trials. 15 , 20 However, it was previously demonstrated that participants from clinical trials differ substantially from real‐world patients. 22 , 23 In patients with metastatic RCC (mRCC) treated with TKIs, 33‐42% of real‐world patients would not be eligible for clinical trials, mostly due to non‐clear cell histology or impaired Eastern Cooperative Oncology Group (ECOG) performance status. 22 Furthermore, some patient categories are underrepresented in clinical trials, such as the elderly or patients with multiple comorbidities. 23 Therefore, it might be challenging to extrapolate these thresholds from clinical trials to real‐world patient cohorts. Some studies have been performed to explore the relationship between exposure and progression‐free survival (PFS) in real‐world patient cohorts, confirming the findings from clinical trials. 24 , 25 However, previous studies in real‐world patients have not investigated the relationship between exposure and both efficacy and toxicity for patients treated with sunitinib.
The aim of this study is to investigate the relationship between sunitinib exposure and treatment outcome, in terms of efficacy and toxicity, in a real‐world patient cohort.
2. METHODS
2.1. Patients
A retrospective observational cohort study was performed in patients with mRCC or metastatic GIST who were treated with sunitinib for at least 2 weeks at the Radboud University Medical Centre, Nijmegen, or at the Jeroen Bosch Hospital, ‘s‐Hertogenbosch, the Netherlands. Patients whose sunitinib sum C trough levels were measured between July 2016 and June 2019 at the Radboud University Medical Centre and who received TDM‐guided dosing were included in this study. This study was approved by the local ethical committee (Commissie Mensgebonden Onderzoek, CMO) of the Radboudumc on 14 May 2019. In accordance with the CMO, informed consent was not obtained, since all data was collected by members of the treating physician team in a retrospective and anonymous manner.
For all patients, clinical data were collected from the electronic patient records. This included data on baseline patient characteristics, diagnosis, disease stage, laboratory results, treatment schedule, dose adjustments during treatment and reason for discontinuation of treatment and comedication interacting with sunitinib (due to inhibition or induction of cytochrome p450 3a4). Furthermore, data on time on treatment (as a surrogate for PFS) and toxicity during treatment were collected. Toxicity was scored according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute, version 4.0. Finally, the results of of all measured sunitinib sum C trough levels were collected.
2.2. Pharmacokinetics
For all patients, blood samples were routinely collected during visits to the outpatient clinic. Blood samples (ethylenediamine tetraacetic acid (EDTA) plasma) were drawn when patients were treated with sunitinib for at least 2 weeks, after reaching steady state pharmacokinetics. In general, TDM samples were collected after patients had been treated for 4, 8 and 12 weeks and thereafter once every 3 months. For each TDM sample, the date and time of last intake of sunitinib and the date and time of the plasma sample collection were recorded. Plasma samples were collected 11‐38 hours after last intake of sunitinib. Sunitinib plasma levels were measured in EDTA plasma using validated high‐performance liquid chromatography tandem mass spectrometry (LC‐MS/MS) similar to the method earlier described by van Erp et al. 26 If the sample was not collected 24 hours after the last intake, the sunitinib sum C trough was calculated using the approach of Wang et al. 27
Since for sunitinib intermittent and continuous dosing schedules were used, dose regimen normalization was required to make comparison between patients possible. 20 Sunitinib shows dose proportional PK. Sunitinib C trough levels of patients who were treated with intermittent dosing were divided by 50 and then multiplied by 37.5. Dose regimen normalization to a continuous dosing schedule was chosen since for the majority of patients C trough levels were measured under a continuous dosing schedule. A threshold for sunitinib exposure of >37.5 ng/mL was used to compare data.
The number of patients under and above target level were calculated. Furthermore, we calculated the success rate of TDM‐guided dose interventions, defined as the percentage of patients achieving target exposure after a dose intervention.
2.3. Exposure‐survival analysis
Since RCC and GIST are two different diseases, the relationship between sunitinib exposure and response was analyzed independently for patients with RCC and for patients with GIST.
Patients were divided into two groups depending on whether or not dose intervention was advised. If dose intervention was advised, patients where further divided into two groups depending on whether or not dose intervention was implemented. Finally, if dose intervention was implemented, patients were grouped determined by whether or not a dose intervention resulted in achieving adequate sunitinib exposure. With each division, median time on treatment was compared between the two groups. Time on treatment was chosen as a surrogate for PFS since in our real‐world patient cohort the Computed Tomography evaluations were not on predefined timepoints, which hampers the determination of PFS.
Next, all patients were stratified according to whether or not they achieved adequate sunitinib sum C trough during treatment, either from the start of treatment or after dose adjustment as advised by the hospital pharmacist. The median PFS was compared between both groups.
2.4. Exposure‐toxicity analysis
Patients were divided into two groups depending on the occurrence of sunitinib‐induced toxicity leading to dose reduction, which was defined as toxicity requiring dose reduction as determined by the treating physician. For each individual patient without toxicity, the geometric mean of all available sunitinib sum C trough levels was calculated. This was compared to the geometric mean of sunitinib sum C trough levels prior to and after dose reduction in patients with toxicity. Since this data was not normally distributed, sunitinib sum C trough in these groups was described with the median and range.
Furthermore, the individual sunitinib sum C trough levels (each level regarded independently) directly prior to dose reductions were compared to all other sunitinib sum C trough levels where after no dose reduction due to toxicity was required.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). Patient characteristics and plasma trough levels were described using descriptive statistics.
The median time on treatment in the different groups, depending on whether or not dose intervention was advised, implemented and/or successful, was analysed by Kaplan‐Meier survival analysis and was compared with the log rank test. Furthermore, the PFS of patients who did or did not achieve adequate C trough during treatment was also analyzed by Kaplan‐Meier survival analysis and compared with the log rank test. An outcome with a P value less than 0.05 was considered to be statistically significant.
Sunitinib sum C trough in patients with or without sunitinib‐induced toxicity leading to dose reduction was compared using the nonparametric Mann‐Whitney U test. An outcome with a P value less than 0.05 was considered to be statistically significant.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
3.1. Patients
A total of 71 patients was included, of whom 53 had RCC and 18 had GIST. The median (range) age was 62 (24‐80) years and 77% of patients were male. Of patients with RCC, most patients had clear‐cell histology (72%) and an intermediate risk prognostic score (51%). Patients with RCC and GIST were treated with sunitinib for a median duration of 31 (2‐264) weeks and 66 (7‐570) weeks, respectively. An overview of patient characteristics, including the ECOG performance status and the localisation of metastases, is presented in Table 1. During treatment with sunitinib, patients could switch from intermittent dosing to continuous dosing or vice versa. Most of the sunitinib sum C trough levels were measured when patients were treated with the continuous dosing schedule (71%).
TABLE 1.
Patient characteristics
| RCC | GIST | Overall | |
|---|---|---|---|
| Patients (n) | 53 | 18 | 71 |
| Gender (n (%)) | |||
| Male | 42 (79) | 13 (72) | 55 (77) |
| Female | 11 (21) | 5 (28) | 16 (23) |
| Age (median (range)) in years | 62 (28‐80) | 61 (24‐73) | 62 (24‐80) |
| Weight (mean (range)) in kg | 83 (48‐132) | 88 (60‐127) | 84 (48‐132) |
| ECOG performance status (n (%)) | |||
| 0 | 10 (20) | 2 (11) | 12 (17) |
| 1 | 31 (61) | 15 (83) | 46 (67) |
| 2 | 10 (20) | 1 (6) | 11 (16) |
| Unknown | 2 (4) | 0 (0) | 2 (3) |
| Localisation of metastases (n (%)) | |||
| Lymph nodes | 26 (49) | 4 (22) | 30 (42) |
| Pulmonary | 27 (51) | 0 (0) | 27 (38) |
| Liver | 10 (19) | 7 (39) | 17 (24) |
| Peritoneum | 6 (11) | 8 (44) | 14 (20) |
| Abdomen | 15 (28) | 11 (61) | 26 (37) |
| Central nervous system | 1 (2) | 0 (0) | 1 (1) |
| Soft tissue | 12 (23) | 2 (11) | 14 (20) |
| Bone | 15 (28) | 2 (11) | 17 (24) |
| IMDC prognostic score (n (%)) | |||
| Good | 11 (21) | NA | NA |
| Intermediate | 27 (51) | ||
| Poor | 9 (17) | ||
| Unknown | 6 (11) | ||
| Histology (n (%)) a | |||
| Clear‐cell | 38 (72) | NA | NA |
| Papillary | 7 (13) | ||
| Chromophobe | 2 (4) | ||
| Other | 8 (15) | ||
| Mutation (n (%)) b | |||
| c‐KIT exon 11 | NA | 8 (44) | NA |
| c‐KIT exon 9 | 2 (11) | ||
| c‐KIT exon 13 | 2 (11) | ||
| PDGFR | 3 (17) | ||
| Other | 2 (11) | ||
| Wildtype | 2 (11) | ||
| Unknown | 1 (6) | ||
| Number of previous lines of systemic treatment (median (range)) | 0 (0‐4) | 1 (1‐4) | 0 (0‐4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GIST, gastrointestinal stromal tumour; IMDC, International Metastatic RCC Database Consortium; NA, not applicable; RCC, renal cell carcinoma.
For histological subtype, some patients had a mixed subtype.
Some patients had multiple mutations.
3.2. Pharmacokinetics
An overview of the results of TDM measurements and dose interventions in our total patient cohort is shown in Figure 1. Based on the sunitinib plasma levels, 39.5% of patients had adequate sunitinib exposure with the starting dose, 39.5% of patients were underdosed and 21% of patients were overdosed. Dose intervention during treatment was advised in 43 of 71 patients (61%). The advised dose intervention was implemented in 25 of 43 patients (58%). If dose intervention was implemented, 92% of patients reached adequate C trough. There was a variety of reasons for nonadherence to the proposed dose recommendation. In 7 of 18 patients, dose intervention could not be implemented due to toxicity. In another 5 out of 18 patients dose intervention was not implemented because both clinical evaluation and radiological investigation revealed response to treatment despite low sunitinib sum C trough levels. In three patients (17%), treatment with sunitinib was discontinued before dose intervention could be implemented. In two of these patients, this was due to rapid disease progression. In the other patient, sunitinib was discontinued due to a combination of toxicity, requiring admission to the hospital and rapidly progressive disease. Finally, in three patients (17%) dose intervention could not be implemented due to combined reasons.
FIGURE 1.
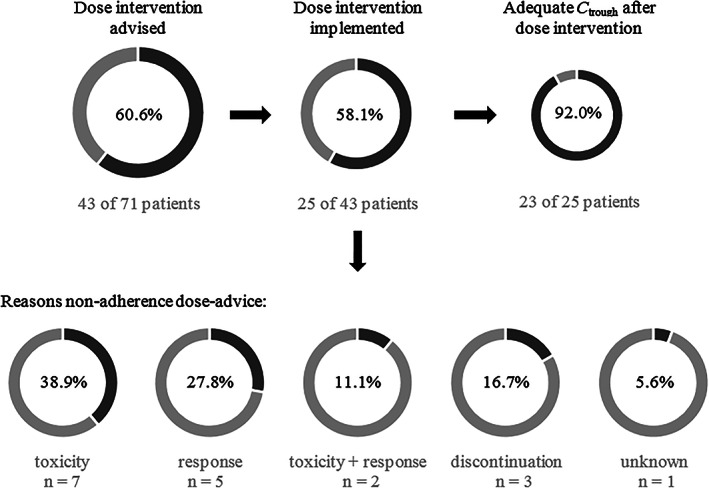
Overview of TDM‐guided dosing. Abbreviations: C trough, plasma trough concentration; TDM, therapeutic drug monitoring
A total number of 217 sunitinib serum samples were available, with a median of two samples per patient (range 1‐19). The dose regimen normalized median (range) sunitinib sum C trough in patients with RCC and GIST were 46 (16‐92) and 45 (21‐63) ng/mL, respectively. In patients with RCC, only 18 of 53 patients (34%) had adequate sunitinib exposure from the start of treatment. However, with TDM‐guided dose adjustments as advised by the hospital pharmacist, this number increased to a total of 39 patients (74%). Adequate C trough was reached after a median (range) of 1 (1‐5) measurement. For patients with GIST, 10 of 18 patients (56%) had adequate sunitinib exposure from the start of treatment. With TDM‐guided dosing, a total of 12 patients (67%) reached adequate C trough after a median of 2 (1‐5) measurements. These results are shown in Table 2.
TABLE 2.
Sunitinib treatment and sample characteristics
| RCC | GIST | Overall | |
|---|---|---|---|
| Sunitinib starting dose (n (%)) | |||
| 12.5 mg | 0 (0) | 1 (6) | 1 (1) |
| 25 mg | 7 (13) | 0 (0) | 7 (10) |
| 37.5 mg | 14 (26) | 17 (94) | 31 (44) |
| 50 mg | 32 (60) | 0 (0) | 32 (45) |
| Sunitinib starting dosing schedule (n (%)) | |||
| Continuous dosing | 14 (26) | 18 (100) | 32 (45) |
| Intermittent dosing | 39 (74) | 0 (0) | 34 (55) |
| Duration of treatment with sunitinib in weeks (median (range)) | 31 (2‐264) | 65.5 (7‐570) | 38 (2‐570) |
| Number of samples per patient (median (range)) | 2 (1‐9) | 4 (1‐19) | 2 (1‐19) |
| Patients who reach adequate C trough (n (%)) | 39 (74) | 12 (67) | 51 (72) |
| C trough in ng/mL (median (range)) | 46 (16‐92) | 45 (21‐63) | 46 (16‐92) |
| TDM# reaching adequate C trough (median (range)) | 1 (1‐5) | 2 (1‐3) | 1 (1‐5) |
Abbreviations: C trough, plasma trough concentration; GIST, gastrointestinal stromal tumour; RCC, renal cell carcinoma; TDM, therapeutic drug monitoring.
3.3. Exposure‐response relationship
Since the number of patients with GIST was small (n = 18), the exposure‐response relationship was only evaluated for patients with RCC. In 2 of 53 patients with RCC, dose intervention was not implemented due to response to treatment as confirmed by radiological investigation. CT scan was considered to be superior to TDM measurement in these patients and therefore they were not included in the following analyses.
For the total group of 51 RCC patients, median time on treatment was 29 weeks. In Table 3, median time on treatment in patients with RCC is shown depending on whether or not dose intervention was advised, implemented and/or successful. Whether or not dose intervention was advised did not affect time on treatment, with a median time on treatment in patients with or without dose advice of 30 (2‐264) weeks and 22 (3‐208) weeks, respectively (P = 0.307). If a dose intervention was advised, the intervention was implemented in 70% of patients. In those patients, a trend towards a longer time on treatment was observed, with a median time on treatment of 44 (3‐264) weeks, compared to 18 (2‐168) weeks in patients in whom dose intervention was not implemented (P = 0.288). Once implementation of the dose advice resulted in adequate C trough levels (91%) median time on treatment was significantly higher, 55 (8‐264) weeks vs 5 (3‐7) weeks (P < 0.001).
TABLE 3.
Exposure‐response relationship in RCC patients depending on whether or not dose intervention was advised, implemented and/or successful
| Number of patients | Percentage | PFS in weeks (median (range)) | P value | ||
|---|---|---|---|---|---|
| Dose adjustment advised | No | 18 | 35 | 22 (3‐208) | 0.307 |
| Yes | 33 | 65 | 30 (2‐264) | ||
| Dose intervention implemented | No | 10 | 30 | 18 (2‐168) | 0.288 |
| Yes | 23 | 70 | 44 (3‐264) | ||
| Adequate C trough after dose intervention | No | 2 | 9 | 5 (3‐7) | <0.001* |
| Yes | 21 | 91 | 55 (8‐264) | ||
Statistically significant.
Abbreviations: C trough, plasma trough concentration; PFS, progression‐free survival.
Figure 3 shows time on treatment in patients with RCC depending on whether or not they reach adequate sunitinib sum C trough during treatment, either from the start of treatment or after dose intervention. In patients who achieve adequate C trough during treatment, time on treatment was higher compared to patients who did not achieve adequate C trough, 32 (3‐264) weeks vs 15 (2‐168) weeks, but results did not reach statistical significance (P = 0.244).
FIGURE 3.
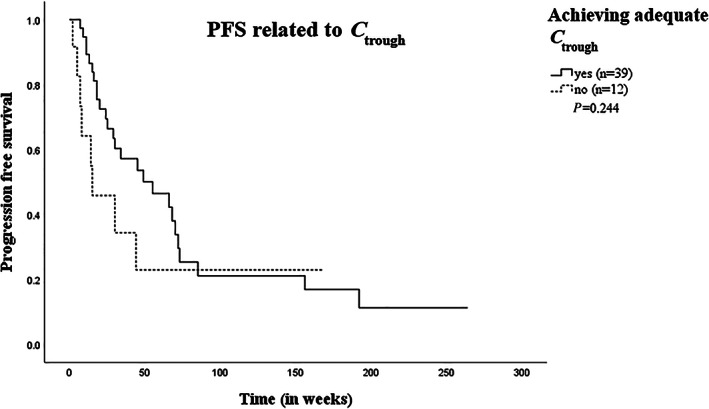
Kaplan‐Meier curve of PFS in RCC patients who did or did not achieve adequate sunitinib sum C trough during treatment. Kaplan‐Meier plot of PFS (in weeks) for RCC patients who did (n = 39, solid line) or did not (n = 12, dashed line) achieve adequate sunitinib sum C trough levels during treatment. Abbrevations: C trough, plasma trough concentration; PFS, progression‐free survival; RCC, renal cell carcinoma
3.4. Exposure‐toxicity relationship
During each patient visit and measurement of sunitinib sum C trough, toxicity was scored. An overview of the observed toxicities at the same timepoints as the collection of each of the 217 sunitinib samples is shown in Table 4. The most frequently observed toxicities were gastrointestinal disorders (such as nausea or vomiting), skin‐ and subcutaneous disorders (eg, hand‐foot syndrome), haematologic toxicity, fatigue and hypertension.
TABLE 4.
Overview of observed toxicities at the time of sunitinib C trough sampling
| Toxicity | Any grade a | Grade ≥ 2 | ||
|---|---|---|---|---|
| Number of reported toxicities | Percentageb | Number of reported toxicities | Percentageb | |
| Gastrointestinal disorders | 120 | 55 | 36 | 17 |
| Skin‐ and subcutaneous disorders (eg hand‐foot syndrome) | 72 | 33 | 25 | 12 |
| Haematological toxicity | 49 | 23 | 14 | 6 |
| Fatigue | 51 | 24 | 18 | 8 |
| Hypertension | 44 | 20 | 30 | 14 |
| Increased liver enzymes | 44 | 20 | 5 | 2 |
| Musculoskeletal disorders | 22 | 10 | 3 | 1 |
| Hypothyroidism | 10 | 5 | 6 | 3 |
| Respiratory disorders | 6 | 3 | 2 | 1 |
| Proteinuria | 1 | 0.5 | 1 | 0.5 |
According to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 4.0.
bPercentage is calculated over a total of 217 obsevation moments.
In our real‐world patient cohort, 29 of 71 patients (41%) experienced sunitinib‐induced toxicity addressed by a dose reduction, as determined by the treating physician. The dose regimen normalized sunitinib sum C trough was significantly higher in patients with toxicity prior to dose reduction compared to patients without toxicity (median 60 (16‐151) vs 44 (21‐89) ng/mL; P < 0.001). After dose reduction, sunitinib sum C trough in patients with toxicity was comparable to patients without clinically relevant toxicity (44 (14‐68) vs 44 (21‐89) ng/mL; P = 0.488). Sunitinib sum C trough in patient with or without sunitinib‐induced toxicity leading to dose reduction is shown in Figure 2. The median (range) time on sunitinib until dose reduction due to toxicity was required was 9.5 (2‐189) weeks.
FIGURE 2.
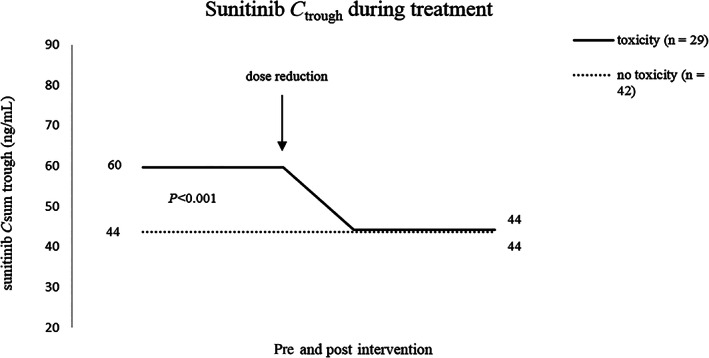
Dose regimen normalized sunitinib sum C trough in patients with and without sunitinib‐induced toxicity leading to dose reduction. Abbrevations: C trough, plasma trough concentration
When considering the individual sunitinib sum C trough levels prior to dose reduction, the median C trough level measured directly before dose reduction (n = 37, 17%) was higher compared to the median of all other sunitinib samples whereafter no dose reduction due to toxicity was required (n = 180, 83%) (67 (16‐161) vs 42 (7‐89) ng/mL; P < 0.001).
4. DISCUSSION
In the current study we investigated the relationship between sunitinib exposure and both efficacy and toxicity in a real‐world patient cohort. All metastatic RCC and GIST patients in this study received TDM‐guided dosing as part of routine patient care. We found a significantly higher sunitinib exposure in patients with sunitinib‐induced toxicity, necessitating dose reductions. After dose reductions, sunitinib exposure was comparable to that of patients without the need of a dose reduction. Furthermore, after implementation of TDM‐guided dose advice, the percentage of patients that reached adequate sunitinib exposure increased to 92%.
In the current study, observed toxicities were similar to toxicity observed in clinical trials and toxicity recently reported in a real‐world patient cohort of patients with GIST who were treated with sunitinib. 1 , 20 , 28 Sunitinib sum C trough was significantly higher in patients with sunitinib‐induced toxicity prior to dose reduction compared to patients without sunitinib‐induced toxicity requiring dose reductions (median 60 vs 44 ng/mL; P < 0.001). Interestingly, however, the dose regimen normalized median sunitinib sum C trough of patients with sunitinib‐induced toxicity leading to dose reduction (60 ng/mL) in our study was lower compared to thresholds for toxicity that were previously reported (>75 ng/mL). 20 , 29 , 30 Similarly, median PFS in patients with RCC in our cohort (31 weeks) was slightly shorter compared to that previously reported in clinical trials (8.3‐11 months). 31 , 32 This could possibly be explained by the fact that patients in our patient cohort differed from the patients included in clinical trials. In the phase III trial of sunitinib in patients with RCC, 62% of patients had an ECOG performance status of 0, compared to only 20% in our patient cohort. 31 Patients with an ECOG performance status of 2 were not included in the trials, while 20% of our patients with RCC had an ECOG performance status of 2. Furthermore, only 15% of patients with RCC in our cohort had a good risk prognostic score, compared to 38% of patients in the clinical trial, while 21% of our patients had a poor risk prognostic score, compared to only 6% of patients in the trial. 31 Another explanation for the lower threshold for toxicity might be that dose reductions in clinical trials are subject to instructions specified in the protocol. In real‐world patients, however, dose might be reduced at an earlier stage, for example due to multiple grade 1‐2 toxicities. Previously, a therapeutic window for sunitinib sum C trough of 37.5‐75 ng/mL for continuous dosing and 50‐87.5 ng/mL for intermittent dosing has been established. 12 , 15 , 20 Based on our results, there is no need to adjust the lower limit of the therapeutic window. However, considering the fact that sunitinib‐induced toxicity leading to dose reduction was associated with a median sunitinib exposure of 60 ng/mL, the upper limit of the therapeutic window might be adjusted for real‐world patients. Based on our results and taking into account the dose‐proportional PK, a therapeutic window for sunitinib exposure in real‐world patients of 37.5‐60 ng/mL for continuous dosing and 50‐80 ng/mL for intermittent dosing might be suggested.
In our patient cohort, sunitinib exposure was outside the therapeutic window and therefore dose intervention was advised in 61% of patients, which indicates that only 39% of patients had adequate sunitinib exposure with the standard dosage. This corresponds with previously studies by Lankheet et al., in which 48‐51% of patients had adequate sunitinib levels with standard treatment. 17 , 33 If dose intervention was advised and thereafter implemented, up to 92% of patients reached adequate sunitinib exposure, confirming the previously reported high success rate of TDM‐guided dosing in treatment with sunitinib. 18
In our cohort, the number of patients with GIST was small and therefore the relationship between exposure and efficacy could not be investigated in these patients. For patients with RCC, there was a trend towards a longer time on treatment in patients who achieved adequate sunitinib exposure during treatment, with a median time on treatment of 32 weeks compared to 15 weeks in patients not achieving adequate sunitinib exposure, although the difference was not statistically significant (P = 0.244). Houk et al. previously compared PFS in RCC patients with a high or low sunitinib exposure, though they did not report numerical PFS. 15 However, the Kaplan‐Meier curve of Houk et al. for patients with mRCC and a high sunitinib exposure is comparable to the Kaplan‐Meier curve of patients who achieved adequate sunitinib exposure in our patient cohort, suggesting that our patients were generally well dosed. The inability to show a significant improvement in PFS with TDM‐guided dosing could most likely be explained by the limited number of patients in this study, especially the number of patients not achieving adequate sunitinib exposure (n = 12, 24%). These patients did not achieve adequate sunitinib exposure due to a variety of reasons (eg, the inability to increase the dose due to toxicity, because the patient was responding to treatment despite low sunitinib sum C trough levels, or since sunitinib treatment was discontinued before dose intervention could be implemented).
TDM‐guided dosing for imatinib, sunitinib and pazopanib is nowadays considered routine patient care in the Netherlands and is being implemented on a nationwide level. 19 However, the data presented in this study are at least in part from a period in which TDM‐guided dosing was performed on the initiative of the treating physician in selected cases, for example in patients not responding to treatment or in patients experiencing toxicity. This could potentially have resulted in selection bias of patients who were over‐ or underdosed and could have affected our results.
This study has several other limitations. First, the number of patients described in this study was relatively small. This may have contributed to the inability to show a significant improve in time on treatment with TDM‐guided dosing, especially since time to progression is influenced by many other factors as well (such as histological subtype of RCC and the International Metastatic RCC Database Consortium prognostic score). 34 Furthermore, the number of patients required to show a significant difference in time on treatment was not calculated in advance and CT evaluations were not performed at predefined timepoints. Second, this was an observational cohort study of real‐world patients, performed in a retrospective manner instead of a superior randomised controlled prospective clinical trial. In particular, toxicity was not always reported according to the CTCAE criteria. The determination of the grade of toxicity was challenging and was subject to the interpretation of the researchers. Clinically relevant toxicity was defined as toxicity requiring dose reduction, which could be accurately collected from patient records. However, dose reductions in real life are based on the interpretation of the treating physician, as opposed to strict regulations in clinical trials. However, despite the limitations, this is the first study investigating the relationship between sunitinib exposure and both efficacy and toxicity in a real‐world patient cohort.
Randomised controlled trials focusing on the efficacy of TDM are lacking. However, in the Netherlands, there is ongoing research in TDM‐guided dosing for TKIs. A large multicenter prospective study is currently being performed in which the efficacy and feasibility of TDM‐guided dosing for up to 23 TKIs is being investigated (www.trialregister.nl; NTR 6866). 35 Furthermore, TDM‐guided dosing is being implemented on a nationwide level for patients with solid tumours treated with imatinib, sunitinib and pazopanib in an infrastructure allowing future expansion for other oral oncolytics with enough evidence for TDM‐guided dosing (the TUNE project [grant no. 11575] funded by the Dutch Cancer Society).
In conclusion, we have shown that sunitinib exposure in real‐world patients with RCC or GIST is significantly higher in patients experiencing sunitinib‐induced toxicity leading to dose reduction compared to patients without toxicity. The threshold for toxicity, however, was lower compared to that previously reported in patients in clinical trials. Based on our results, a therapeutic window for sunitinib exposure in real‐world patients of 37.5‐60 ng/mL for continuous dosing and 50‐80 ng/mL for intermittent dosing might be suggested. Especially in a palliative setting, preserving the right balance between benefit of treatment and the occurrence of toxicity (and therewith tolerance to treatment) is of utmost importance. Therefore, TDM‐guided dosing can be an elegant and feasible tool to improve the treatment of these patients.
ACKNOWLEDGEMENTS
This study was part of the TUNE project (grant no. 11575) funded by the Dutch Cancer Society (KWF Kankerbestrijding).
CONTRIBUTORS
KW: conception and design, collection of data from patient records, drafting of the manuscript, final approval. SK: conception and design, revising the manuscript, final approval. WG: conception and design, revising the manuscript, final approval. SM: treatment of included patients, revising the manuscript, final approval. CvH: treatment of included patients, revising the manuscript, final approval. TS: collection of data from patient records, treatment of included patients, revising the manuscript, final approval. NE: conception and design, drafting and revising the manuscript, final approval. ID: conception and design, treatment of included patients, drafting and revising the manuscript, final approval.
COMPETING INTERESTS
There are no competing insterests to declare.
Westerdijk K, Krens SD, van der Graaf WTA, et al. The relationship between sunitinib exposure and both efficacy and toxicity in real‐world patients with renal cell carcinoma and gastrointestinal stromal tumour. Br J Clin Pharmacol. 2021;87:326–335. 10.1111/bcp.14332
Since this was a retrospective observational cohort study and patients were treated according to routine patient care, there was no principal investigator
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal‐cell carcinoma. N Engl J Med. 2013;369(8):722‐731. 10.1056/NEJMoa1303989 [DOI] [PubMed] [Google Scholar]
- 2. Demetri GD, Garrett CR, Schoffski P, et al. Complete longitudinal analyses of the randomized, placebo‐controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170‐3179. 10.1158/078-0432.CCR-11-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501‐513. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 4. Committee for Medicinal Products for Human Use (CHMP) EMA . Sunitinib assessment report 2019 [Available from: https://www.ema.europa.eu/en/documents/variation-report/sutent-h-c-687-ii-0070-epar-assessment-report-variation_en.pdf.
- 5. US Food and Drug Administration , Center for Drug Evaluation and Research. Sunitinib clinical pharmacology and biopharmaceutics review. 2006 [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021938_S000_Sutent_BioPharmR.pdf.
- 6. Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet‐derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327‐337. [PubMed] [Google Scholar]
- 7. Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet‐derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16‐24. 10.1200/JCO.2005.02.574. Epub Dec 5. [DOI] [PubMed] [Google Scholar]
- 8. Sun Y, Li J, Yang X, Zhang G, Fan X. The alternative 2/1 schedule of Sunitinib is superior to the traditional 4/2 schedule in patients with metastatic renal cell carcinoma: a meta‐analysis. Clin Genitourin Cancer. 2019;21(18):30603‐30607. [DOI] [PubMed] [Google Scholar]
- 9. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959‐1968Epub Mar 11. DOI: 10.01s6/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 10. de Wit D, Guchelaar HJ, den Hartigh J, Gelderblom H, van Erp NP. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today. 2015;20(1):18‐36. 10.1016/j.drudis.2014.09.007 Epub Sep 22 [DOI] [PubMed] [Google Scholar]
- 11. Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305‐325. 10.1007/s40262-014-0137-2 [DOI] [PubMed] [Google Scholar]
- 12. Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765‐776. 10.1002/cpt.787 Epub 2017 Sep 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groenland SL, Mathijssen RHJ, Beijnen JH, Huitema ADR, Steeghs N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur J Clin Pharmacol. 2019;7(10):19‐2704. [DOI] [PubMed] [Google Scholar]
- 14. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27(19):3141‐3147. 10.1200/JCO.2008.20.4818 Epub 2009 May 18 [DOI] [PubMed] [Google Scholar]
- 15. Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta‐analysis. Cancer Chemother Pharmacol. 2010;66(2):357‐371. 10.1007/s00280-009-1170-y Epub 2009 Dec 5 [DOI] [PubMed] [Google Scholar]
- 16. Suttle AB, Ball HA, Molimard M, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909‐1916. doi: 10.038/bjc.2014.503. Epub Oct 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lankheet NA, Kloth JS, Gadellaa‐van Hooijdonk CG, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumours. Br J Cancer. 2014;110(10):2441‐2449. 10.1038/bjc.2014.194 Epub Apr 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lankheet NAG, Desar IME, Mulder SF, et al. Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol. 2017;83(10):2195‐2204. 10.1111/bcp.13327 Epub 2017 Jul 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westerdijk K, Desar IME, Steeghs N, van der Graaf WTA, van Erp NP. Imatinib, sunitinib and pazopanib: from flat‐fixed dosing towards a pharmacokinetically guided personalized dose. Br J Clin Pharmacol. 2019;28(10):14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25‐35. 10.1200/JCO.2005.02.194 Epub Nov 28 [DOI] [PubMed] [Google Scholar]
- 21.Committee for Medicinal Products for Human Use (CHMP) EMA. Sunitinib summary of product characteristics 2019. [Available from: https://www.ema.europa.eu/en/documents/product-information/sutent-epar-product-information_en.pdf.
- 22. Mitchell AP, Harrison MR, Walker MS, George DJ, Abernethy AP, Hirsch BR. Clinical trial participants with metastatic renal cell carcinoma differ from patients treated in real‐world practice. J Oncol Pract. 2015;11(6):491‐497. 10.1200/JOP.2015.004929 Epub 2015 Sep 1 [DOI] [PubMed] [Google Scholar]
- 23. Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383‐1389. doi: 10.200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 24. Farag S, Verheijen RB, Martijn Kerst J, Cats A, Huitema AD, Steeghs N. Imatinib pharmacokinetics in a large observational cohort of gastrointestinal stromal tumour patients. Clin Pharmacokinet. 2017;56(3):287‐292. doi: 10.1ss007/s40262‐016‐0439‐7. [DOI] [PubMed] [Google Scholar]
- 25. Verheijen RB, Swart LE, Beijnen JH, Schellens JHM, Huitema ADR, Steeghs N. Exposure‐survival analyses of pazopanib in renal cell cfvarcinoma and soft tissue sarcoma patients: opportunities for dose optimization. Cancer Chemother Pharmacol. 2017;80(6):1171‐1178. doi: 10.007/s00280‐ss17‐3463‐x. Epub 2017 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Erp NP, de Wit D, Guchelaar HJ, Gelderblom H, Hessing TJ, Hartigh J. A validated assay for the simultaneous quantification of six tyrosine kinase inhibitors and two active metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;937:33‐43. 10.1016/j.jchromb.2013.08.013 Epub Aug 17 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31(5):579‐584. 10.1097/FTD.0b013e3181b2c8cf [DOI] [PubMed] [Google Scholar]
- 28. Den Hollander D, Van der Graaf WTA, Desar IME, Le Cesne A. Predictive factors for toxicity and survival of second‐line sunitinib in advanced gastrointestinal stromal tumours (GIST). Acta Oncol. 2019;58(11):1648‐1654. doi: 10.080/0284186X.2019.1637017. Epub 2019 Jul 26 [DOI] [PubMed] [Google Scholar]
- 29. Noda S, Otsuji T, Baba M, et al. Assessment of Sunitinib‐induced toxicities and clinical outcomes based on therapeutic drug monitoring of Sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):350‐358. 10.1016/j.clgc.2015.01.007 Epub Jan 21 [DOI] [PubMed] [Google Scholar]
- 30. Takasaki S, Kawasaki Y, Kikuchi M, et al. Relationships between sunitinib plasma concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma. Int J Clin Oncol. 2018;23(5):936‐943. 10.1007/s10147-018-1302-7 Epub 2018 Jun 2 [DOI] [PubMed] [Google Scholar]
- 31. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med. 2007;356(2):115‐124. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 32. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in advanced renal‐cell carcinoma. N Engl J Med. 2018;378(14):1277‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lankheet NA, Knapen LM, Schellens JH, Beijnen JH, Steeghs N, Huitema AD. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit. 2014;36(3):326‐334. 10.1097/FTD.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 34. Ko JJ, Xie W, Kroeger N, et al. The international metastatic renal cell carcinoma database consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first‐line targeted therapy: a population‐based study. Lancet Oncol. 2015;16(3):293‐300. 10.1016/S470-2045(14)71222-7 Epub 2015 Feb 12 [DOI] [PubMed] [Google Scholar]
- 35. Netherlands Trial Register NTR6866 . Therapeutic drug monitoring for oral anti‐cancer drugsTC=6866. Accessed 15 January 2019. http://www.trialregister.nl/trialreg/admin/rctview.asp?.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


