FIGURE 1.
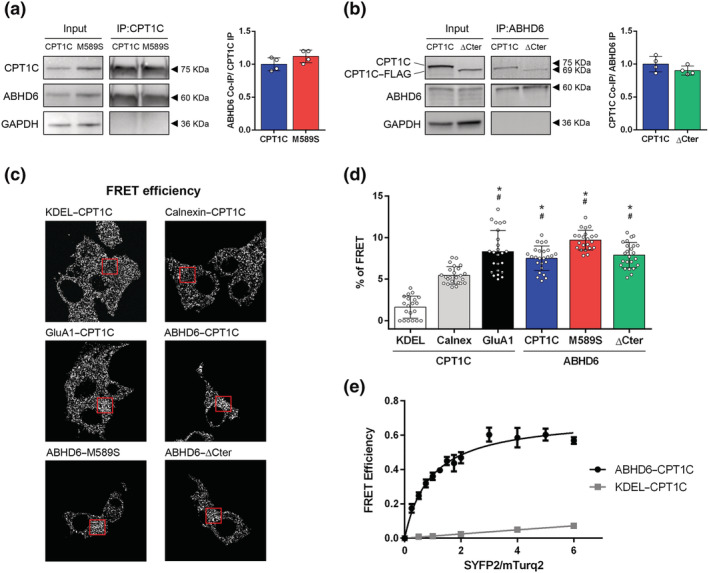
ABHD6 interacts with CPT1C. (a) Co‐immunoprecipitation of ABHD6‐SYFP2 in HEK‐293T cells using CPT1C antibody for CPT1C and M589S (n = 4). (b) Co‐immunoprecipitation of CPT1C and ΔCter in HEK‐293T cells using GFP–Trap for ABHD6–mTurq2 (n = 4). Proteins were detected in whole lysate (input) and immunoprecipitated (IP) samples. (c) Representative pictures of FRET efficiency. (d) FRET efficiency of CPT1C–SYFP2 interaction with mTurq2–KDEL and mTurq2–Calnexin as negative FRET interactions, and CPT1C–SYFP2 with GluA1–mTurq2 as a positive FRET interaction. ABHD6–mTurq2 interaction with CPT1C–SYFP2, M589S–SYFP2, and ΔCter‐SYFP2. Percentage of FRET was measured by the increase in donor intensity after photobleaching (n = 25). (e) FRET sensitized emission of CPT1C with either ABHD6 or KDEL in living cells. Assays were performed 48 h post transfection in HEK‐293T cells expressing ABHD6–mTurq2 or mTurq2–KDEL and with increasing amounts of the cDNA for CPT1C–SYFP2. FRET saturation curves for both protein pairs were obtained by monitoring SYFP2 fluorescence emission at 530 nm after excitation of mTurq2 at 420 nm and subtracting values obtained with cells expressing the same amount of donor protein. Means ± SD (n = 5). *P < 0.05, significantly different from KDEL‐CPT1C; # P < 0.05, significantly different from Calnexin‐CPT1C; one‐way ANOVA followed by Bonferroni's post hoc correction
