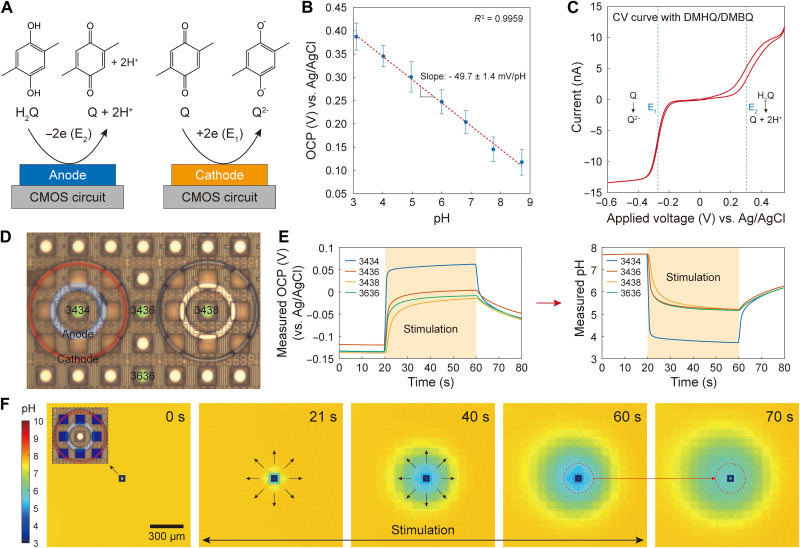
Fig. 2. pH manipulation with quinone chemistry and proton diffusion.
(A) Illustration of redox reactions of DMHQ or H2Q for brevity and DMBQ (or Q). The oxidation reaction generates protons, while the reduction reaction generates quinone dianions (Q2−), which serve as base. (B) OCP versus pH calibration. The OCP measured by our OCP sensors with the circular Pt electrodes has a linear relationship to pH with an experimentally determined sensitivity of −49.7 ± 1.4 mV/pH. This calibration was conducted by measuring OCP of various pH buffer solutions. Only the slope of this calibration line is used in converting OCP to pH (for details, see Materials and Methods). (C) Cyclic voltammogram at a single circular Pt electrode (which in this case is used not in the OCP sensing mode but in the potentiostat mode) with a scan range from −0.6 to 0.5 V and a scan rate of 20 mV/s. (D) A positive current is injected into the anodic ring around pad “3434” (i.e., the Al pad located at row 34 and column 34), with the results presented in parts (E) and (F). (E) The OCP measured with the OCP sensor at pad 3434 (left) and its conversion to pH (right) using the calibration result of part (B) (see Materials and Methods). (F) Spatiotemporal pH imaging during the stimulation of the single anodic ring around pad 3434. The temporal evolution of the pH heatmap shows a radial diffusion of protons generated by the anodic stimulation. The ceiling height is about 39 μm.