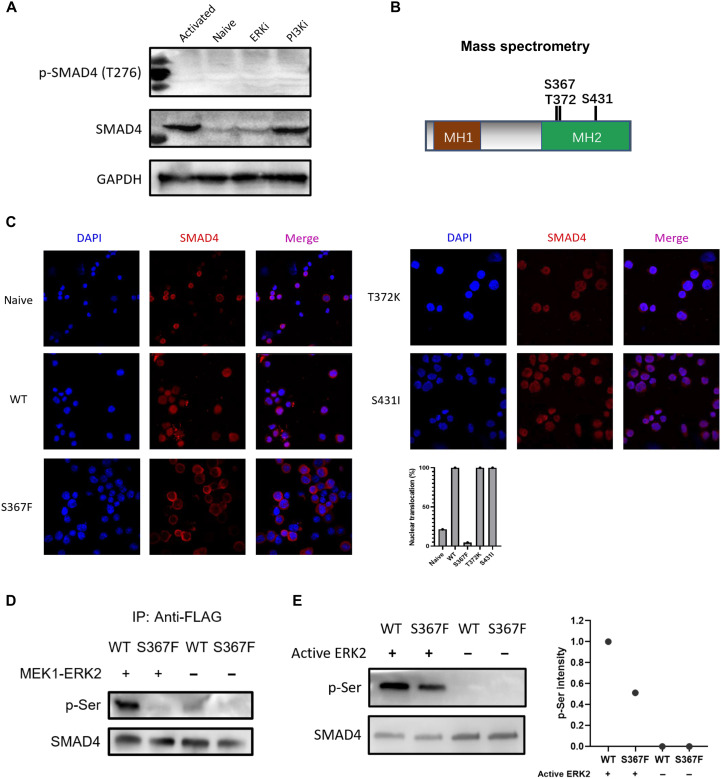
Fig. 6. SMAD4 phosphorylation at Ser367 by ERK regulates SMAD4 nuclear translocation.
(A) Expression levels of total SMAD4 and phosphorylated SMAD4 at Thr276 in naive and αCD3/CD28 activated CD8+ T cells (in the presence of DMSO control, PI3K inhibitor, ERK inhibitor, respectively). ERKi, ERK1/2 inhibitor; PI3Ki, PI3K inhibitor. (B) Three candidate serine/threonine phosphorylation sites (Ser367, Thr372, and Ser431) on SMAD4. Cell lysates from αCD3/CD28 activated CD8+ T cells were subjected to anti-SMAD4 purification via immunoprecipitation followed by mass spectrometry analysis. (C) Immunofluorescence staining data of SMAD4 (red; Alexa Fluor 594) in naive or αCD3/CD28 activated CD8+ T cells infected with retrovirus harboring WT Smad4 or the three mutant Smad4 gene (24 hours after retrovirus infection). Statistic data of SMAD4 nuclear translocation ratio were determined by manually counting the percentage of cells containing higher SMAD4 staining intensity in the nucleus versus cytoplasm in three representative fields. (D) Co-overexpressed MEK1 and ERK2 phosphorylated SMAD4 at Ser367 site. FLAG-SMAD4-WT or FLAG-SMAD4-S367F and MEK1-ERK2 were cotransfected into HEK-293T cells. Immunoprecipitation by anti-FLAG and Western blotting were carried out. (E) Western blotting results of in vitro phosphorylation assay by using recombinant active ERK2. FLAG-SMAD4-WT and FLAG-SMAD4-S367F were immunoprecipitation-purified with anti-FLAG affinity gel, washed by 3× FLAG peptide. Phospho-serine (p-Ser) levels (relative to total SMAD4) were quantified by ImageJ software. These experiments were repeated two or three times with consistent results.