Abstract
IMPORTANCE:
Aggressive fluid resuscitation remains a cornerstone of the Surviving Sepsis Campaign (SSC) guidelines, but there is growing controversy regarding the recommended 30 mL/kg IV fluid dosage. It is contended that, in selected patients, this volume confers an increased risk of volume overload without either concomitant benefit or strong evidence in support of the recommended IV fluid dosage.
OBJECTIVES:
Assessment of practice patterns and their impact on patient outcomes following the surviving sepsis guidelines for fluid resuscitation.
DESIGN:
Large, multisite retrospective cohort study.
SETTING AND PARTICIPANTS:
The retrospective study included all adult patients who presented to the emergency department at one of 19 different Mayo Clinic sites throughout the Midwest, Southeast, and Southwest from August 2018 to November 2020 with suspected sepsis.
MAIN OUTCOMES AND MEASURES:
Eight-thousand four-hundred fourteen patients suspected to have sepsis were assessed regarding fluid resuscitation and outcomes among patients receiving 30 mL/kg IV fluid dosing compared with patients who did not. Patient demographics and clinical information were collected via electronic health records. Patients were divided into two cohorts: those who received 0–29.9 mL/kg of IV fluid and those who received 30.0+ mL/kg of IV fluid. Statistical analyses were performed to evaluate the impact of fluid dose on in-hospital death, 30-day mortality, ICU admission after diagnosis, dialysis initiation after diagnosis, ventilator use, vasopressor use, as well as ICU and hospital length of stay.
RESULTS:
We observed lower in-hospital mortality and 30-day mortality risk in the 30+ mL/kg dosing group. Increased fluid dosage did, however, carry a much greater chance of ICU admission. Most patients (72% after propensity score weighting) in our population received less than 30 mL/kg fluid (based on ideal body weight).
CONCLUSIONS AND RELEVANCE:
IV fluid dosing for sepsis resuscitation greater than 30 mL/kg was associated with decreased risk of in-hospital mortality, 30-day mortality, and reduced risk of requiring mechanical ventilation. Our data does ultimately seem to support the SSC recommendation.
Keywords: fluid dose, in-hospital mortality, intensive care unit length of stay, outcomes, resuscitation, sepsis
Sepsis, a life-threatening, dysregulated host response to infection, contributes to an estimated 30–50% of inpatient deaths in the United States (1). Its occurence rate has actually increased in the past two decades across the country, possibly due in part to increased recognition following the Surviving Sepsis Campaign (SSC) (2, 3). IV fluid dosing for sepsis resuscitation has always been a matter of debate. The international Surviving Sepsis guidelines provide limited evidence behind their recommendation for 30 mL/kg (ideal bodyweight) fluid resuscitation within 3 hours of recognition of sepsis, with the original dosage partly suggested by an earlier retrospective analysis of a sepsis database in 2013 (4). In fact, the latest Surviving Sepsis Guidelines 2021 has downgraded the fluid resuscitation protocol (30 mL/kg within 3 hr of recognition of sepsis/septic shock) from strong to weak due to low quality of evidence (5). A substantial contingent of physicians in critical care have voiced concern over the SSC fluid resuscitation guidelines, prompting a debate about outright retiring SSC (6). Although it should be noted that the fluid dose is only one of over 90 recommendations from the SCC guidelines. The American College of Emergency Physicians has even issued an official statement that SSC guidelines advocate an inappropriate and dangerous one-size-fits-all approach to sepsis (7). Thus, it is vital that the critical care community engage in formal studies to obtain evidence related to this controversial topic.
The present study was designed to assess practice patterns with regard to fluid dose as well as outcomes of those patients that received 30+ mL/kg fluid dosing with the outcomes of those who did not. The intent of the study design was to assess the potential associations of fluid dosing with respect to the SSC guidelines of 30 mL/kg of IV fluid on outcomes in patients admitted to the hospital with sepsis. We hypothesized that we would see no significant differences in mortality between cohorts and increased incidence of mechanical ventilation in the + 30 mL/kg of IV fluid.
METHODOLOGY
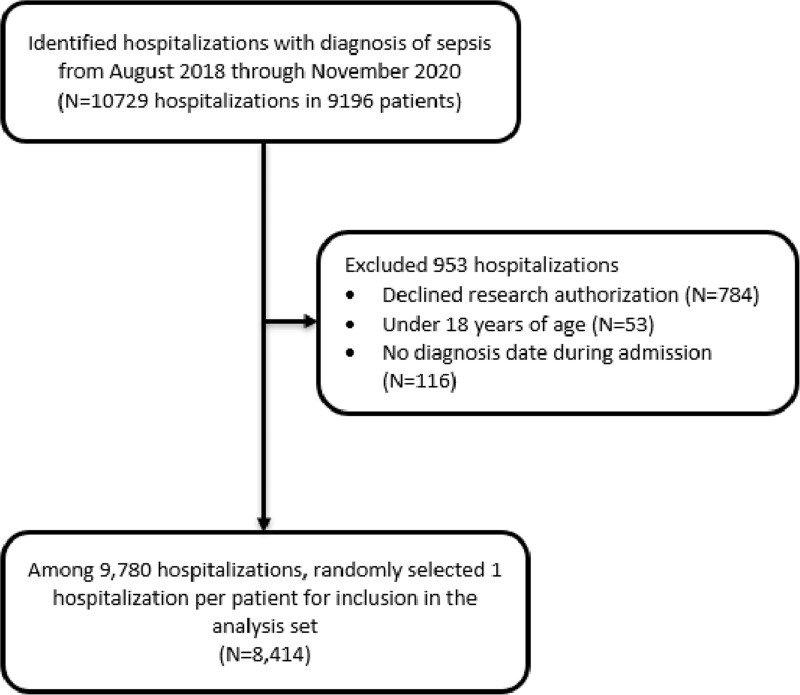
This retrospective study included all adult patients (n = 8,414) who presented to the emergency department at one of 19 different Mayo Clinic sites throughout the Midwest, Southeast, and Southwest from August 2018 to November 2020 suspected to have sepsis. Patients were excluded if they were under 18 years old at the time of presentation to the emergency department. If a patient had more than one emergency department visit with a diagnosis of sepsis during the study period, then we randomly selected one visit per patient for inclusion in the study. We randomly selected the visit as opposed to always selecting the first or second visit to avoid biasing the study toward better or worse outcomes, as a patient likely has a greater chance of death on the second admission. Including multiple admissions from the same patient would overcomplicate the analysis because although the admissions may not be related, we would expect the patient’s characteristics from each admission to have highly correlated data. If we included all of the visits and did not account for the within-patient correlation, it would give more weight to those patients who had repeated admissions, giving more weight to patients who are sicker. We excluded any patient whose date of diagnosis was missing (Fig. 1). A sample size estimation was not performed, as the data were pulled from a large database of sepsis patients formed prior to the design of this study.
Figure 1.
Inclusion of patients.
The electronic health record was used to obtain information on patient demographics (age at diagnosis, sex, and race), clinical information known at the time of diagnosis (body weight, comorbidities, and ICU admission prior to diagnosis), information on fluid therapy for treatment of sepsis, and patient outcomes. Patient demographics, clinical information, and hospital information were summarized according to IV fluid dose in the first 6 hours after diagnosis of sepsis (0–29.9 vs 30.0+ mL/kg; based on ideal body weight). Time of diagnosis was determined by either time of antibiotic administration or time of lactate greater than 2.0 mmol/L detected. Time of draw, not result, was used as the time of diagnosis, so this should be quite close to the time of antibiotic administration. We chose whichever time came first. The primary outcome of the study was in patient mortality, while the secondary outcomes included hospital length of stay, 30-day mortality (death within 30 d of sepsis diagnosis), ICU admission after diagnosis, ICU length of stay, new onset dialysis after diagnosis, mechanical ventilator use, and vasopressor use. Information on fluid type was also collected for use in a separate analysis to follow.
Mayo Clinic Institutional Review Board granted an exemption from need for approval for our study on September 3, 2020. The application number for our study is 20-008691, and the study title is “Assessing Healthcare quality metrics in sepsis, severe sepsis and septic shock patients in Mayo Clinic Enterprise.” The need for informed consent was waived by our Institutional Board Review. Procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
STATISTICAL ANALYSIS
Logistic regression was used to evaluate the impact of fluid dose on in-hospital death, 30-day mortality, ICU admission after diagnosis, dialysis initiation after diagnosis, ventilator use, and vasopressor use. Cox proportional hazards regression was used to examine the impact of fluid dose on hospital length of stay (censoring at date of death for those who died in the hospital) and on ICU length of stay.
To control confounding, propensity score methods were used to improve balance in the patient characteristics of the two fluid dose groups. Propensity score is defined here as the conditional probability of receiving 30 or more mL/kg of fluid for diagnosis of sepsis given a set of covariates known at the time of diagnosis. A multivariable logistic regression model with 30 or more mL/kg of fluid as our dependent variable and all the patient demographics, clinical information, and hospital information shown in Table 1 as covariates were used to estimate the propensity score (Pi) for each patient (i). Stabilized inverse probability weights (wi) were then calculated as wi = P*/Pi for those who received 30 or more mL/kg of fluid and wi = (1–P*)/(1–Pi) for those who received less than 30 mL/kg of fluid where P* is the proportion of patients in our cohort who received 30 or more mL/kg of fluid. The level of balance between the two-dose groups was assessed using weighted standardized mean differences (SMDs), where we considered SMDs less than 10% to indicate negligible imbalance between groups and SMDs greater than 20% indicated substantial imbalance between groups. All p values were two-sided without adjustment for multiple testing. All statistical analyses were performed using R (Version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
TABLE 1.
Prediagnosis Characteristics According to the Fluid Dose, Before and After Propensity Score Weighting
| Patient Characteristics | Before PS-Weighting | After PS-Weighting | ||||
|---|---|---|---|---|---|---|
| Fluid 0–29.9 mL/kg (n = 6,074) | Fluid ≥ 30 mL/kg (n = 2,340) | Unweighted SMD, % | Fluid 0–29.9 mL/kg (N* = 6,082) | Fluid ≥ 30 mL/kg (N* = 2,317)* | Weighted SMD, % | |
| Demographic information | ||||||
| Age at diagnosis (yr) | 70 ± 16 | 68 ± 17 | 12.3 | 70 ± 16 | 70 ± 16 | 0.2 |
| Sex (male) | 58.6 | 45.2 | 27.1 | 54.7 | 54.1 | 1.2 |
| Race | ||||||
| American Indian/Alaskan Native | 0.8 | 0.6 | 3.2 | 0.8 | 0.4 | 5.1 |
| Asian | 1.5 | 2.6 | 7.2 | 1.9 | 2.1 | 1.3 |
| Black | 2.7 | 2.7 | 0.1 | 2.7 | 2.8 | 0.3 |
| Native Hawaiian/Pacific Islander | 0.1 | 0.2 | 2.4 | 0.1 | 0.2 | 1.1 |
| White | 92.2 | 90.4 | 6.5 | 91.8 | 91.1 | 2.2 |
| Other/unknown | 2.6 | 3.5 | 5.6 | 2.7 | 3.4 | 4.4 |
| Comorbidities | ||||||
| Chronic obstructive pulmonary disease | 12.7 | 11.2 | 4.9 | 12.3 | 11.9 | 1.1 |
| Hypertension | 44.6 | 37.6 | 14.3 | 42.5 | 41.8 | 1.4 |
| Chronic kidney disease | 22.8 | 16.2 | 16.7 | 20.9 | 20.4 | 1.2 |
| Diabetes | 24.8 | 19.4 | 13.1 | 23.2 | 22.5 | 1.7 |
| Coronary artery disease | 21.2 | 17.3 | 10.0 | 20.1 | 19.5 | 1.5 |
| Congestive heart failure | 18.9 | 10.3 | 24.6 | 16.4 | 15.7 | 2.0 |
| Obesity | 31.9 | 21.0 | 24.9 | 28.8 | 28.1 | 1.4 |
| Dialysis | 3.9 | 3.0 | 4.7 | 3.6 | 3.8 | 0.8 |
| Hospital information | ||||||
| Hospital type (destination) | 60.9 | 65.2 | 8.8 | 62.3 | 63.5 | 2.5 |
| In ICU prior to diagnosis | 16.9 | 20.2 | 8.5 | 17.8 | 17.5 | 0.9 |
| Lactate measurement at diagnosis | 40.9 | 61.9 | 43.0 | 46.9 | 47.5 | 1.3 |
N* = sum of propensity score weights, PS = propensity score, SMD = standardized mean difference.
Numerical characteristics are given as mean ± sd, while categorical characteristics are given as the percentage of patients.
RESULTS
We identified 8,414 patients during the study period who were admitted to the hospital with the diagnosis of sepsis; of these, 6,074 (72%) were given 0 to 29.9 mL/kg of fluid after diagnosis of sepsis and 2,340 (28%) were given 30 or more mL/kg of fluid in the first 6 hours after diagnosis of sepsis. Patients’ prediagnosis characteristics before and after propensity score weighting are summarized in Table 1 and show substantial differences (SMD > 20%) between fluid dose groups in sex, comorbidities (congestive heart failure [CHF] and obesity), and type of diagnosis (lactate measurement vs antibiotic). Patients who received less than 30 mL/kg of fluid were more likely to be male, have CHF and obesity and were less likely to have a lactate measurement at diagnosis. After propensity score weighting, all differences in prediagnosis characteristics were considered negligible (all SMDs ≤ 5.1%).
In our original (unweighted) cohort, 7.6% died in the hospital (primary outcome), 7.3% among those who received less than 30 mL/kg of fluid and 8.5% among the group who received 30 or more mL/kg of fluid. See Table 2 for a summary of outcomes according to fluid level.
TABLE 2.
Association of Fluid Dose (≥ 30.0 vs < 30.0 mL/kg) With Outcomes: Before and After Propensity Score Weighted Analysis
| Outcomes | Fluid < 30 mL/kg (n = 6,074) | Fluid ≥ 30 mL/kg (n = 2,340) | Unweighted Analysis | p | PS-Weighted Analysis | p |
|---|---|---|---|---|---|---|
| OR or HR (95% CI) | OR or HR (95% CI) | |||||
| Primary outcome | ||||||
| In-hospital mortality | 442 (7.3%) | 198 (8.5%) | 1.18 (0.99–1.40) | 0.067 | 0.80 (0.66–0.96) | 0.020 |
| Secondary outcomes | ||||||
| Hospital length of stay after diagnosis, d | 4 (3–7) | 4 (3–7) | 0.98 (0.93–1.03)a | 0.37 | 1.02 (0.97–1.08) | 0.47 |
| Death within 30 d of diagnosis | 891 (14.7%) | 382 (16.3%) | 1.13 (1.00–1.29) | 0.058 | 0.81 (0.71–0.93) | 0.003 |
| ICU admission after diagnosis | 1,115 (22.1%), n = 5,046 | 681 (36.5%), n = 1,867 | 2.02 (1.80–2.27) | < 0.001 | 1.79 (1.60–2.01) | < 0.001 |
| Started dialysis after diagnosis | 36 (0.6%), n = 5,840 | 11 (0.5%), n = 2,270 | 0.79 (0.3–1.49) | 0.48 | 1.34 (0.73–2.38) | 0.33 |
| Mechanical ventilator use | 521 (8.6%) | 215 (9.2%) | 1.08 (0.91–1.27) | 0.41 | 0.79 (0.66–0.94) | 0.008 |
| Vasopressor use | 494 (8.1%) | 279 (11.9%) | 1.53 (1.31–1.78) | < 0.001 | 1.11 (0.94–1.30) | 0.22 |
HR = hazard ratio, OR = odds ratio, PS = propensity score.
aFor hospital length of stay after diagnosis, a HR > 1.00 represent better outcomes for patients who received 30 or more mL/kg of fluid compared with those who received < 30 mL/kg of fluid. For categorical outcomes, ORs > 1.00 represent worse outcomes for patients who received 30 or more mL/kg of fluid compared with those who received < 30 mL/kg of fluid.
However, in our propensity score weighted analysis controlling for potentially confounding information known prior to diagnosis, we observed a lower risk of in-hospital death among those in the higher fluid group (odds ratio, 0.80; 95% CI, 0.66–0.96; p < 0.05). We also observed lower risk of 30-day mortality (p < 0.05) and lower risk of being placed on a mechanical ventilator (p < 0.05) with 30 or more mL/kg of fluid compared with less than 30 mL/kg of fluid on our propensity score weighted analyses. But patients who received more than 30 mL/kg of fluid were substantially more likely to require ICU admission after diagnosis (1.79; 95 % CI, 1.60–2.01; p < 0.01).
DISCUSSION
Regarding our aim to assess practice patterns, we noted that a majority of the patients (72% after propensity score weighting) in our population received less than 30 mL/kg fluid (based on ideal body weight). This suggested to us that among physicians in our sample sites, the treatment protocol used was quite a contrast to the SSC guidelines for fluid dosage. Regarding our aim to determine patient outcomes by fluid dose, contrary to our hypothesis, we saw lower risk of both in-hospital mortality and 30-day mortality in these patients. Fluid resuscitation creates a risk of pulmonary edema, but—also contrary to our hypothesis—there was lower risk of mechanical ventilation in the high fluids group (after propensity score weighting). Increased fluid dosage did, however, carry a much greater risk of ICU admission. Other markers of benefit—hospital length of stay, ICU length of stay, initiation of dialysis after diagnosis, and vasopressor use—were unaffected by the fluid dose.
Our study found a clear mortality benefit from the 30 mL/kg fluid dose. We surmise that our findings of mortality benefit are intimately linked with the increased likelihood of ICU admission. The higher fluid dose in these patients perhaps signifies how sick they were to begin with. A patient with refractory shock bound for the ICU would likely receive more intense, prolonged periods of resuscitation with more fluid. Conversely, a physician would be more likely to stop fluid resuscitation early if the patient responded well initially. This may in fact be a premature cessation of fluid resuscitation that could explain the higher mortality seen in our less than 30 mL/kg fluid group. We expected to see a pattern of more cautious fluid resuscitation in patients with CHF and chronic kidney disease (CKD), but instead our data revealed a similar proportion of CHF and CKD patients in both fluid groups. This suggests that physicians’ fluid dosage likely depends on the patient’s response. A CHF patient with refractory shock is still more likely to receive more fluid compared with a patient without CHF who responds to the first fluid bolus.
A recent meta-analysis revealed a dearth of high-quality studies to guide fluid resuscitation (8). Our study has a number of strengths. We have over 8,000 patients, creating enormous power for our analysis. Furthermore, our study has excellent generalizability, as it draws from 19 sites, both academic and community, from across multiple regions including the Southeast, Southwest, and Midwestern United States. Our study did target 30 mL/kg per the SSC recommendation, but we chose to extend the window of treatment from 3 to 6 hours. Historically, we tend to give less fluids to patient with comorbidities. Even when we give fluid, the rate of administration is slow. With the basics trial published recently, there was not any difference between slow and fast group. Thus, to capture a wider number of patients, especially with comorbidities and with the overall hypothesis of total fluid resuscitation rather than rapidity, we chose 6 hours as compared with 3 hours.
Our study shares a significant limitation with all observational studies of titratable interventions: simultaneity bias. Simultaneity bias occurs when a variable on the right-hand side of the equation can influence a variable on the left-hand side of the equation while simultaneously the variable on the left-hand side of the equation influences the variable on the right-hand side. In medicine, this occurs when an intervention influences outcome while simultaneously the predicted outcome or response to treatment can influence the dosage of treatment. Our patients with more severe hypotension will respond well to larger doses of fluid, but patients with more severe hypotension are more likely to be given larger doses of fluid. This creates a chicken-and-egg effect in which it is difficult to determine a true cause and effect relationship. This limitation is inherent in all observational studies of titratable interventions and signifies the need for future randomized trials of fluid resuscitation (9). Another limit of our study is survivorship bias. Patients who died within the first 6 hours of sepsis recognition would have less time to receive fluid and would be less likely to receive more than 30 mL/kg. Propensity score methods were used to improve balance in the patient characteristics of the two fluid dose groups including age at diagnosis, sex, race, body weight, comorbidities, and ICU admission prior to diagnosis, but not illness severity, which is a limitation. This is a retrospective analysis, so although random sampling was used for patient selection, it may not eliminate sampling bias completely. We do not have clear reasons—for example, recorded measures of fluid responsiveness—for why some patients received more fluid than others. Also, there was no standardization of volume, rate, type, or mode of delivery of fluid resuscitation among our patients, all of which could have significant impact on outcome. Sepsis fluid protocols, when present, were not standardized across sites. Particularly, lack of information on infusion rate is a salient limitation, as previous studies have shown better survival with quicker rates (10). Unfortunately, over 90% of the patients in the study are White, which is not representative of the U.S. population.
With a titratable intervention that has a continuous range of dosage, one would assume a continuous range of benefit and harm, along which range the optimum dose can be found. This analysis actually serves as pilot study for our group. Our next step is to create an analysis with the fluid dose and outcomes split into quintiles in an attempt to identify the optimum dose. Our initial study was built specifically to investigate the utility of the most widely spread fluid resuscitation paradigm, the SSC guidelines. Our data does ultimately seem to support SSC recommendation.
CONCLUSIONS
IV fluid dosing for sepsis resuscitation greater than 30 mL/kg was associated with a statistically significant decreased risk of in-hospital mortality, 30-day mortality, and decreased risk of requiring mechanical ventilation, but it was associated with increased risk of ICU admission.
ACKNOWLEDGMENTS
We would like to thank all the coauthors involved in this study for their useful contributions. We acknowledge that our work has not been presented/published previously as an abstract. Dr. Govero would like to thank his fiancé for her love and patience.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Liu V, Escobar GJ, Greene JD, et al. : Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–92 [DOI] [PubMed] [Google Scholar]
- 2.Mayr FB, Yende S, Angus DC: Epidemiology of severe sepsis. Virulence 2014; 5:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, et al. : Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu V, Morehouse JW, Soule J, et al. : Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann Am Thorac Soc 2013; 10:466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, et al. : Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e1143 [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Rhodes A, Evans LE; Steering and Executive Committee of the Surviving Sepsis Campaign: Rebuttal from Drs Levy, Rhodes, and Evans. Chest 2019; 155:19–20 [DOI] [PubMed] [Google Scholar]
- 7.Yealy DM, Mohr NM, Shapiro NI, et al. : Early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: A consensus-based task force report. Ann Emerg Med 2021; 78:1–19 [DOI] [PubMed] [Google Scholar]
- 8.Meyhoff TS, Møller MH, Hjortrup PB, et al. : Lower vs higher fluid volumes during initial management of sepsis: A systematic review with meta-analysis and trial sequential analysis. Chest 2020; 157:1478–1496 [DOI] [PubMed] [Google Scholar]
- 9.Leisman DE: The Goldilocks effect in the ICU-when the data speak, but not the truth. Crit Care Med 2020; 48:1887–1889 [DOI] [PubMed] [Google Scholar]
- 10.Hu B, Chen JCY, Dong Y, et al. : Effect of initial infusion rates of fluid resuscitation on outcomes in patients with septic shock: A historical cohort study. Crit Care 2020; 24:137. [DOI] [PMC free article] [PubMed] [Google Scholar]