Table 3.
Reactions with ethyl diazoacetate.
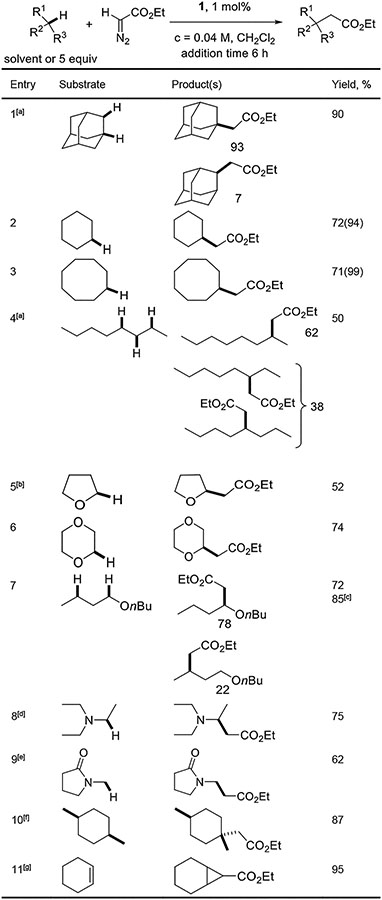
|
Catalyst (1 mol%), entries 1, 3, 4: CH2Cl2 (6.5 mL), substrate (2.5 mmol, 5.0 equiv), add ethyl diazoacetate (0.5 mmol, 1.0 equiv, in 6.5 mL CH2Cl2) in 6 hours at RT. Entries 2, 6, 7, 10: substrate used as solvent (10 mL, 118–234 equiv). Yields are isolated yields. [a] Isolated as a mixture of isomers. [b] Scale: 1 mmol, THF solvent (20 mL, 246 mmol). [c] Yield determined by 1H NMR spectroscopy with an internal standard. [d] Catalyst 2 (3 mol%), Et3N (15 mL, 108 mmol), CHCl3 (15 mL), add ethyl diazoacetate (10 mmol) at 70 °C in 3 h. [e] Catalyst 2 (3 mol%), NMP (5 mL, 52 mmol), CHCl3 (5 mL), add ethyl diazoacetate (1 mmol in CHCl3) at 60 °C in 12 h. [f] cis-1,4-Dimethylcyclohexane (7 equiv), catalyst 2 (2 mol%). Contains less than 5% of another product arising from insertion in a secondary C-H bond. [g] Major product. About 10/1 ratio of cyclopropanation/C-H insertion observed.
