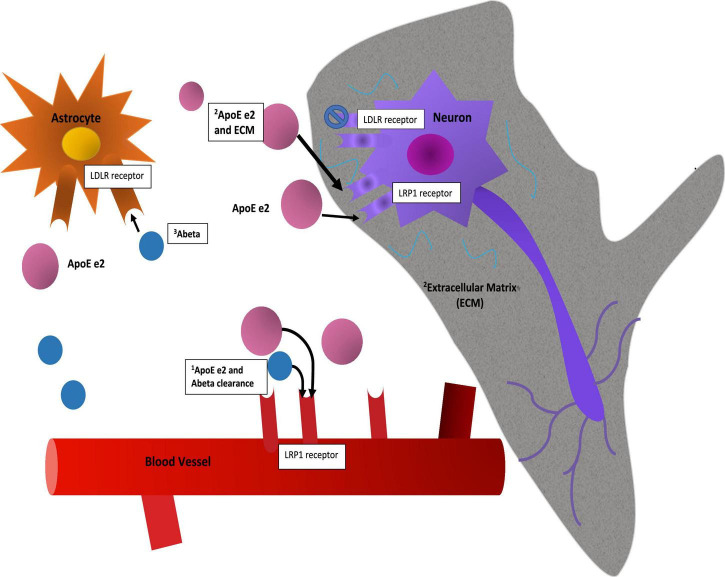
FIGURE 1.
Mechanisms. This figure is a speculative but neurobiologically plausible mechanistic model of how two established molecular properties of the e2 isoform, namely its protein abundance and its low affinity for the LDLR receptor, might provide initial stages of neuroprotection. While mRNA levels of the isoforms appear to be equivalent (Conejero-Goldberg et al., 2014), post translational differences due to isoform related susceptibility to cleavage or other degradation related processes result in full-length protein level differences (Riddell et al., 2008; Mahley, 2016). The mechanistic interpretation of such differences in brain are not established: Speculatively, protein abundance may have advantageous effects in and of itself by way of clearance of Ab, including via the BBB, or delivery of cholesterol to neurons for synaptic maintenance. As a caveat to this, the e4 isoform may be “toxic,” so simply increasing abundance might not be advantageous. Second, neuroprotective effects may be associated with reduced binding at a primary cellular ApoE receptor, LDLR, with the corollary that more ApoE e2 is more available at other receptors, such as LRP1 or ApoER2 and in parallel, allow other ligands to stimulate the LDLR receptor. These and other upstream (e.g., promoter variants) and downstream factors (e.g., isoform specific differences in lipidation, microglial activation, LTP reduction, etc.) are discussed elsewhere.