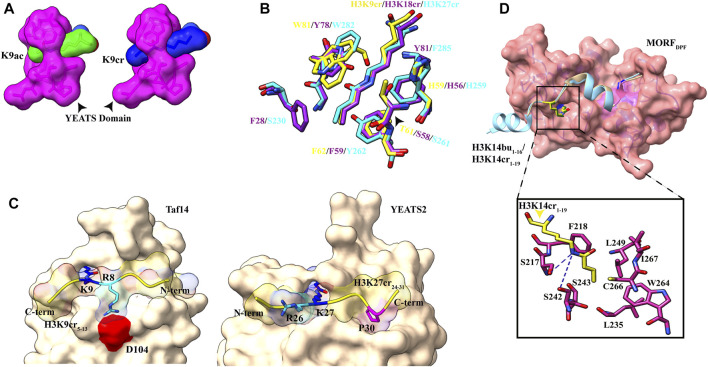
FIGURE 4.
Readout of acyl marks by YEATS and DPF domains. (A) Longer chain of the crotonyl group (blue) can be accommodated in the Taf14 YEATS domain (magenta). (PDB ID: 5D7E and 5IOK). (B) Superimposition of residues involved in interaction among YEATS domains of different proteins: YEATS2 YEATS domain–H3K27cr (cyan; PDB ID: 5IQL), Taf14 YEATS domain–H3K9cr (yellow; 5IOK), and AF9 YEATS domain–H3K18cr (purple; 5HJD). (C) Opposite orientation of H3 peptides across Taf14 (PDB ID: 5IOK) and YEATS2 (PDB ID: 5IQL) proteins. In the Taf14–H3K9cr5–13complex, R8 (cyan) interacts with aspartate at 104th position. In YEATS2–H3K27cr24–31 complex, R26 (cyan) is facing away from the YEATS domain but proline at 30th position (magenta) makes contacts with the hydrophobic pocket. (D) Top: overall structure of the DPF domain of MORF protein with H3K14bu1–16 (wheat) and H3K14cr1–19 (cyan). Bottom: close-up view of amino acids involved in interaction between crotonylated lysine (yellow) and MORFDPF protein (magenta) (PDB ID: 6OIE and 5B76).