Figure 1.
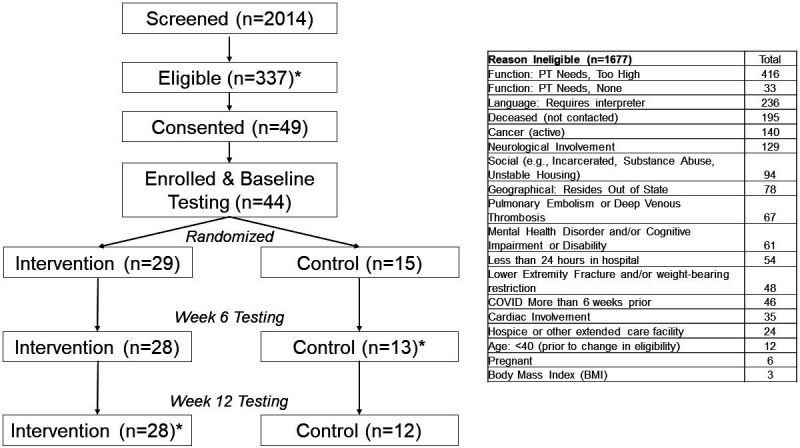
Trial flow chart reasons for ineligibility are provided in the table at the right side of the figure. If ineligibility was determined through chart review (eg, patient had active cancer or was deceased), the patient was not contacted by the study team. Five individuals signed the informed consent form but did not complete baseline testing due to already being healthy and no longer perceiving benefit (n=2), losing interest/no longer wanting to participate and being lost to follow-up. *Most common reasons for ineligibility were that the patient had physical therapy (PT) needs that were too high (n=416), the patient had language barriers (n=236), the patient was deceased (n=195), the patient had active cancer (n=140) or the patient had neurological involvement (n=129). At week 6, one participant in the control group completed patient-reported outcome measures but did not complete functional testing. At week 12, one participant in the intervention group completed patient-reported outcome measures but did not complete functional testing.
