Abstract
The increased prevalence of β-lactamase is one of the main factors in resistance to β-lactams in Pseudomonas aeruginosa. This study aimed to investigate the prevalence of blaVEB, blaPER, and blaGES genes in β-lactam-resistant P. aeruginosa. We collected 100 non-duplicated clinical isolates of P. aeruginosa and identified them by standard tests. Using disk agar diffusion test, we detected the β-lactam-resistant isolates and extracted the DNAs of the isolates by alkaline lysis method. Then, the prevalence of blaVEB, blaPER and blaGES genes were detected by PCR method. The results were assessed by SPSS 21 software and Chi-square test. Out of 100 isolates, 43% were detected as resistant against at least one of the beta-lactams tested. Piperacillin-tazobactam was the most effective antibiotic, while 39% and 37% of the isolates were resistant to aztreonam and meropenem, respectively. A significant relationship was observed between the resistance to tested antibiotics and the presence of blaVEB, blaGES, and blaPER genes. Among 43 isolates that were resistant to at least one of the tested β-lactams, 93.02%, 83.72%, and 81.39% of them carried blaVEB, blaGES, and blaPER genes, respectively. According to this study and due to high prevalence of β-lactam resistance genes, it is better to check the level of antibiotic resistance and resistance genes for better management of patients with infection caused by this bacterium. Also, high prevalence of class A β-lactamases indicates the significant role of these enzymes in emerging resistance to beta-lactams.
Keywords: Pseudomonas aeruginosa, blaVEB, blaGES, blaPER, beta-lactam resistance
1. Introduction
Among all the bacteria that cause nosocomial infections, gram-negative bacilli are of particular importance, while Pseudomonas aeruginosa has the highest priority among these bacteria [1]. P. aeruginosa is a non-fermenter gram-negative bacillus commonly found in hospitals and plays an important role in the development of different acute and chronic nosocomial infections [1],[2]. The large genome of P. aeruginosa (5.5–7 Mbp) enables high metabolic and environmental changes adaptability in this organism [3]. Multiple virulence factors, simple nutritional requirements, and high antimicrobials resistance rate, make this organism as an extremely dangerous pathogen [4],[5]. Treating infections caused by this bacterium has become a major challenge due to the organism's ability to develop high-level antibiotic resistance, as the World Health Organization (WHO) recently introduced the carbapenem-resistant P. aeruginosa as one of the bacteria needing the new drugs [6]. P. aeruginosa can be resistant to a variety of antibiotics, including aminoglycosides, quinolones, and β-lactams, by different inherently or acquired resistance mechanisms [7]. The β-lactam antibiotics, which contain β-lactam rings in their molecular structure, stop the biosynthesis of bacterial cell walls by targeting penicillin-binding proteins [7]. The annual cost of these antibiotics is approximately $ 15 billion and accounts for 65% of the total antibiotic market, while antibiotic resistance against these classes is increasing [7],[8]. The previous studies conducted in Asian countries in 2019 and 2020 showed that almost 12–39% of Iranian P. aeruginosa isolates and 26–60% of Gram-negative bacilli collected from Saudi Arabia were resistant to different β-lactams [2],[9]. Also, Nasser et al. exhibited that 3–100% of P. aeruginosa isolates collected in the Arab region from 2011 to 2018 were resistant against various β-lactams [10].
Resistance to these drugs can occur by a variety of mechanisms but is mainly due to the production of β-lactamases [9],[11]. As a causative agent of nosocomial infections, β-lactamase producing P. aeruginosa is one of the most harmful organisms to humans [10]. Among four classes of β-lactamases [12], class A beta-lactamase genes can be on plasmid, integron, or chromosome, and have the highest distribution among gram-negative bacteria [13]. Among these enzymes, some beta-lactamases such as Pseudomonas Extended Resistant (PER), Guiana extended-spectrum β-lactamases (GES), and Vietnam Extended Spectrum Beta-lactamase (VEB), which are part of extended-spectrum β-lactamases (ESBLs), are highly prevalent and significant in clinical isolates of P. aeruginosa [14]. The PER β-lactamase hydrolyzes most penicillins and cephalosporins and is mostly found in isolates from Turkey and Mediterranean countries [15],[16]. The VEB enzyme also promotes resistance to ceftazidime, aztreonam, and cefepime and is well inhibited by clavulanate and avibactam [17]. The GES family, as the carbapenemases, are more common in P. aeruginosa isolates and inhibited by clavulanate, tazobactam, avibactam, relebactam, and vaborbactam, however, the GES-1 hydrolyzes ceftazidime better than cefotaxime [18]. The genes encoding these three enzymes are usually carried by transposons and can move between Gram-negative bacteria [19]. Class A enzymes play a significant role in emerging resistance to β-lactams. Many studies have been published on these enzymes and their role in gram-negative bacilli. However, there is an urgent need for further studies on how these enzymes are resistant to clinically important β-lactams. Therefore, due to the great importance of the presence of PER, VEB, and GES β-lactamase encoding genes in the development of resistance to β-lactams, we aimed to evaluate the prevalence of these genes in clinical isolates of P. aeruginosa in this region of Iran.
2. Materials and methods
2.1. Ethical approval statements
Although the clinical samples were transferred to the Department of Medical Microbiology from the laboratories of the hospitals affiliated with the Mazandaran University of Medical Sciences, a written informed permission form was provided by the patients or a close relative, and organizing evidence of each sample was reserved secret. Also, this study was directed in agreement with the Declaration of Helsinki.
2.2. Bacterial isolation and identification
We collected 100 non-repetitive P. aeruginosa isolates from different clinical specimens. The specimens were included urine, sputum, eye secretion, catheter, wound, stool, and blood. These samples were collected from patients hospitalized in 5 therapeutic and educational hospitals affiliated with Mazandaran University of Medical Sciences, Sari, Iran, from 2018 to 2019. The isolates were identified by the standard microbiological and biochemical tests such as gram staining, pigment production on Müller-Hinton agar medium (Merck, Germany), non-fermentation reaction, and growth at 42 °C in triple sugar iron (TSI) agar (Merck), motility, citrate utilization, oxidase test, oxidation/fermentation (OF) test, and colony odor [20]. Then, the bacteria were frozen at −20 °C in trypticase soy broth (TSB) (Merck) containing 10% glycerol until use.
2.3. Antimicrobial susceptibility testing
We used the disk agar diffusion method to determine the antibiotic resistance pattern of the P. aeruginosa clinical isolates according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [21]. In this study, 8 β-lactam antibiotics along with a β-lactam/ β-lactamase inhibitor combination including piperacillin (10 µg), piperacillin-tazobactam (10–110 µg), aztreonam (30 µg), cefepime (30 µg), ceftazidime (30 µg), imipenem (10 µg), meropenem (10 µg) and doripenem (10 µg) (MAST, UK) were used. After 18 h incubation of the cultures at 37 °C, we measured the zone of no bacterial growth around the antibiotic disks by ruler and reported the results as resistant, intermediate resistant, or susceptible. P. aeruginosa ATCC 27853 was chosen as a control strain in antimicrobial susceptibility testing.
2.4. DNA extraction from the bacterial isolates
We used the alkaline lysis method by adding sodium dodecyl sulfate (SDS) and NaOH for the extraction of the genomic DNAs of P. aeruginosa clinical isolates [22]. Briefly, we prepare an extraction solution by dissolving 0.5 g of SDS (Sigma, Germany) and 0.4 g of NaOH (Sigma) in 200 µL of sterile distilled water. Next, 4–6 colonies of pure bacteria were dissolved in 20 µL of this solution in a microtube. Then, the microtube was placed at 95 °C for 10 min and centrifuged for 3 min at 13000 g. Finally, 180 µL of sterile distilled water was added to the microtube and the supernatant was used as the extracted DNA. The OD (Optical Density) of the DNAs was measured using a NanoDrop (ND1000, USA). Also, the extracted DNAs were electrophoresed on 1.5% agarose gel (Wizbiosolutions, South Korea). Lastly, the extracted DNAs were stored in a freezer at −20 °C.
2.5. Detection of resistance genes using PCR
We used the specific primers (Metabion, Germany) including veb-forward-5′-CGACTTCCATTTCCCGATGC-3′ and veb-revers-5′-GGACTCTGCAACAAATACGC-3′ for detection of blaVEB gene with a product size of 642 bp [23], per-forward-5′-ATGAATGTCATTATAAAAGC-3′ and per-revers-5′-TTAATTTGGGCTTAGGG-3′ for detection of blaPER gene with a product size of 933 bp [24], and ges-forward-5′-GTTTTGCAATGTGCTCAACG-3′ and ges-revers-5′-TGCCATAGCAATAGGCGTAG-3′ for detection of blaGES gene with a product size of 387 bp [25] by PCR test. The PCR reaction was carried out in a final volume of 15 µL containing 7.5 µL of PCR Master Mix (Ampliqon, Denmark), 5 pmol of each primer, 5.5 µL of distilled water, and 300 ng of DNA. The initial denaturation step was done at 94 °C for 5 min. Then, 34 cycles of the amplification were performed as follows: Denaturation at 94 °C for 45 sec, annealing step for 20 sec at 63 °C for blaVEB, 55 °C for blaGES, and 52 °C for blaPER, and extension at 72 °C for 25 sec. A final extension step was used at 72 °C for 10 min. Then, the PCR product was electrophoresed on 1% agarose gel (Wizbiosolutiotions) with 1.5 µL of Safe Stain (SinaClon, Iran).
2.6. Statistical analysis
The data was introduced into SPSS software version 22, and the desired results were statistically analyzed using Pearson's Chi-Square test. P-values < 0.05 was considered statistically significant.
3. Results
3.1. Clinical data and isolation of the bacteria
We collected 100 non-duplicated P. aeruginosa isolates from 100 non-repeated inpatients in 5 educational and treatment hospitals including Imam Khomeini Hospital (40 isolates), Razi Hospital (22 isolates), Bu-Ali Sina Hospital (17 isolates), Zare Hospital (11 isolates) and Fatemeh Al-Zahra Hospital (10 isolates) affiliated to Mazandaran University of Medical Sciences, Sari, Iran. Out of 100 isolates, 60 of them were gotten from men. The mean age of women was 47.85 and the mean age of men was 44.76. The bacterial isolates were collected from respiratory samples (37 isolates), urine (26 isolates), wounds (20 isolates), blood (5 isolates), ocular discharge (2 isolates), stool (2 isolates), and catheters (8 isolates). Moreover, among 100 clinical isolates of this study, 53, 13, 6, 6, 5, 5, 4, 3, 2, 2, and 1 isolates were collected from intensive care units (ICUs), emergency, burn ward, operating room and surgery, cardiac care units (CCUs), pediatric ward, internal, men, women, neurology, and oncology wards, respectively.
3.2. Antibiotic resistance pattern of the isolates
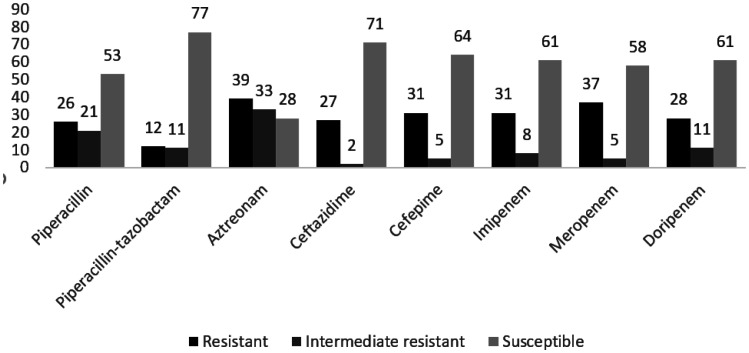
The antibiotic resistance pattern of P. aeruginosa clinical isolates against the 8 antibiotics tested in this study is shown in Figure 1. However, aztreonam was the least effective antibiotic due to a 39% resistance rate, while just 12% of the isolates were resistant against piperacillin-tazobactam. Moreover, 4% of the isolates were resistant to all tested antibiotics. Also, most intermediate resistance rate was shown against aztreonam, while just 2% of the isolates showed this phenotype against ceftazidime.
Figure 1. Antimicrobial resistance pattern of 100 P. aeruginosa clinical isolates in this study.
Also, Table 1 shows the antibiotic susceptibility pattern of the P. aeruginosa clinical isolates regarding the hospital wards in which the bacteria were isolated. According to this data, the highest resistance was related to the bacteria isolated from the burn ward. However, the resistance rates in surgery units and operating rooms were considerable.
Table 1. Number (%) of antibiotic resistant P. aeruginosa clinical isolates considering the hospital ward.
| Hospital wards |
ICU (n = 53) |
Emergency (n = 13) |
Burn (n = 6) |
Surgery (n = 6) |
CCU (n = 5) |
Pediatric (n = 5) |
Internal (n = 4) |
Men (n = 3) |
Women (n = 2) |
Neurology (n = 2) |
Oncology (n = 1) |
| Antibiotics | |||||||||||
| Piperacillin | 15 (28.3) | 4 (30.76) | 3 (50) | 1 (16.66) | - | 1 (20) | 1 (25) | 1 (33.33) | - | - | - |
| Piperacillin-tazobactam | 7 (13.2) | 2 (15.38) | 1 (16.66) | 1 (16.66) | - | - | - | 1 (33.33) | - | - | - |
| Aztreonam | 21 (39.62) | 5 (38.46) | 5 (83.33) | 4 (66.66) | - | - | 1 (25) | 1 (33.33) | 1 (50) | - | 1 (100) |
| Ceftazidime | 16 (30.18) | 4 (30.76) | 2 (33.33) | 2 (33.33) | - | 1 (20) | 1 (25) | 1 (33.33) | - | - | - |
| Cefepime | 18 (33.96) | 3 (23.07) | 4 (66.66) | 3 (50) | - | 1 (20) | 1 (25) | 1 (33.33) | - | - | - |
| Imipenem | 19 (35.84) | 2 (15.38) | 1 (16.66) | 2 (33.33) | 1 (20) | 2 (40) | 2 (50) | 1 (33.33) | - | 1 (50) | - |
| Meropenem | 21 (39.62) | 2 (15.38) | 3 (50) | 3 (50) | 1 (20) | 2 (40) | 2 (50) | 1 (33.33) | 1 (50) | 1 (50) | - |
| Doripenem | 15 (28.3) | 2 (15.38) | 3 (50) | 2 (33.33) | - | 2 (40) | 2 (50) | 1 (33.33) | - | 1 (50) | - |
3.3. Identification of blaVEB, blaGES, and blaPER genes by PCR
In the present study, 40%, 36%, and 35% of P. aeruginosa clinical isolates carried blaVEB, blaGES, and blaPER genes, respectively. Also, according to Table 2, which shows the number of β-lactam resistant isolates containing the studied genes, a significant relationship was observed between the presence of blaVEB, blaGES, and blaPER genes with resistance to the studied beta-lactams (P-value < 0.05). Among the 43 isolates that were resistant to at least one of the tested β-lactams, 40 (93.02%), 36 (83.72%), and 35 (81.39%) isolates carried the blaVEB, blaGES, and blaPER genes, respectively. Out of the 3 blaVEB-negative isolates, one was only susceptible to piperacillin-tazobactam and the other two isolates were only susceptible to ceftazidime. Among the 7 isolates without the blaGES gene, only one was resistant to piperacillin-tazobactam, while 2 isolates were intermediate resistant and 4 isolates were susceptible to this antibiotic. Isolates without the blaPER gene had the same situation. Although the presence of these genes was significantly associated with the development of resistance to the tested β-lactams, this association was slightly weaker for carbapenems, while resistance to doripenem was most strongly associated with the presence of these genes among carbapenems. However, among 43 β-lactam resistant isolates in this study, 34 (79.06%), 33 (76.74%), 31 (72.09%), and 30 (69.76%) isolates contained blaVEB + blaGES, blaVEB + blaPER, blaGES + blaPER, and blaVEB + blaGES + blaPER genes, respectively.
Table 2. Relationship between the presence of blaVEB, blaGES, and blaPER genes in clinical isolates of P. aeruginosa and resistance to beta-lactams.
| Genes |
Number (%) of isolates containing β-lactamase encoding genes |
||||||
|
blaVEB
|
blaGES
|
blaPER
|
|||||
| Antibiotics and antibiotic resistance pattern (number) | positive | negative | positive | negative | positive | negative | |
| Piperacillin | R (26) | 24 (92.3) | 2 (7.69) | 23 (88.46) | 3 (11.53) | 25 (96.15) | 1 (3.84) |
| I (21) | 12 (57.14) | 9 (42.85) | 11 (52.38) | 10 (47.61) | 9 (42.85) | 12 (57.14) | |
| S (53) | 4 (7.54) | 49 (92.45) | 2 (3.77) | 51 (96.22) | 1 (1.18) | 52 (98.11) | |
|
| |||||||
| Piperacillin-tazobactam | R (12) | 11 (91.66) | 1 (8.33) | 11 (91.66) | 1 (8.33) | 11 (91.66) | 1 (8.33) |
| I (11) | 10 (90.9) | 1 (9.09) | 9 (81.81) | 2 (18.18) | 9 (81.81) | 2 (18.18) | |
| S (77) | 19 (24.67) | 58 (75.32) | 16 (20.77) | 61 (79.22) | 15 (19.48) | 62 (80.51) | |
|
| |||||||
| Aztreonam | R (39) | 36 (92.3) | 3 (7.69) | 33 (84.61) | 6 (15.38) | 32 (82.05) | 7 (17.94) |
| I (33) | 4 (12.12) | 29 (87.87) | 3 (9.09) | 30 (90.9) | 3 (9.09) | 30 (90.9) | |
| S (28) | - | 28 (100) | - | 28 (100) | - | 28 (100) | |
|
| |||||||
| Ceftazidime | R (27) | 26 (96.29) | 1 (3.7) | 24 (88.88) | 3 (11.11) | 26 (96.29) | 1 (3.7) |
| I (2) | 2 (100) | - | 2 (100) | - | 1 (50) | 1 (50) | |
| S (71) | 12 (16.9) | 59 (83.09) | 10 (14.08) | 61 (85.91) | 8 (11.26) | 63 (88.73) | |
|
| |||||||
| Cefepime | R (31) | 28 (90.32) | 3 (9.67) | 28 (90.32) | 3 (9.67) | 29 (93.54) | 2 (6.45) |
| I (5) | 5 (100) | - | 4 (80) | 1 (20) | 3 (60) | 2 (40) | |
| S (64) | 7 (10.93) | 57 (89.06) | 4 (6.25) | 60 (93.75) | 3 (4.68) | 61 (95.31) | |
|
| |||||||
| Imipenem | R (31) | 21 (67.74) | 10 (32.25) | 20 (64.51) | 11 (35.48) | 20 (64.51) | 11 (35.48) |
| I (8) | 4 (50) | 4 (50) | 4 (50) | 4 (50) | 4 (50) | 4 (50) | |
| S (61) | 15 (24.59) | 46 (75.4) | 12 (19.67) | 49 (80.32) | 11 (18.03) | 50 (81.96) | |
|
| |||||||
| Meropenem | R (37) | 27 (72.97) | 10 (27.02) | 25 (67.56) | 12 (32.43) | 26 (70.27) | 11 (29.72) |
| I (5) | 3 (60) | 2 (40) | 3 (60) | 2 (40) | 3 (60) | 2 (40) | |
| S (58) | 10 (17.24) | 48 (82.75) | 8 (13.79) | 50 (86.2) | 6 (10.34) | 52 (89.65) | |
|
| |||||||
| Doripenem | R (28) | 23 (82.14) | 5 (17.85) | 21 (75) | 7 (25) | 22 (78.57) | 6 (22.14) |
| I (11) | 6 (54.54) | 5 (45.45) | 6 (54.54) | 5 (45.45) | 6 (54.54) | 5 (45.45) | |
| S (61) | 11 (18.03) | 50 (81.96) | 9 (14.75) | 52 (85.24) | 8 (13.11) | 53 (86.88) | |
Abbreviations: R; resistant, I; intermediate resistant, S; susceptible
Moreover, Table 3 shows the relationship between the presence of the studied genes and the hospital wards of bacterial isolation. While most of the presence of resistance genes was observed in isolates collected from the burn section, no statistically significant relationship was observed in this regard. However, after assessing the relationship between the presence of genes and clinical sample type, we concluded that the presence of blaVEB and blaGES genes had a statistically significant relationship with the type of clinical samples and the highest presence of genes was observed in isolates collected from the wounds and catheters (Table 4).
Table 3. Relationship between the presence of blaVEB, blaGES, and blaPER genes with the hospital ward related to sample collection.
| Hospital wards | No. (%) of isolates carrying the relevant genes in the desired hospital wards |
|||||
|
blaVEB
|
blaGES
|
blaPER
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| ICU (n = 53) | 21 (39.62) | 32 (60.37) | 20 (37.73) | 33 (62.26) | 20 (37.73) | 33 (62.26) |
| Emergency (n = 13) | 5 (38.46) | 8 (61.53) | 5 (38.46) | 8 (61.53) | 4 (30.76) | 9 (69.23) |
| Burn (n = 6) | 4 (66.66) | 2 (33.33) | 5 (83.33) | 1 (16.66) | 5 (83.33) | 1 (16.66) |
| Surgery (n = 6) | 4 (66.66) | 2 (33.33) | 2 (33.33) | 4 (66.66) | 2 (33.33) | 4 (66.66) |
| Pediatric (n = 5) | 1 (20) | 4 (80) | - | 5 (100) | 1 (20) | 4 (80) |
| CCU (n = 5) | - | 5 (100) | - | 5 (100) | - | 5 (100) |
| Internal (n = 4) | 3 (75) | 1 (25) | 2 (50) | 2 (50) | 2 (50) | 2 (50) |
| Men (n = 3) | 1 (33.33) | 2 (66.66) | 1 (33.33) | 2 (66.66) | 1 (33.33) | 2 (66.66) |
| Women (n = 2) | - | 2 (100) | - | 2 (100) | - | 2 (100) |
| Neurology (n = 2) | - | 2 (100) | - | 2 (100) | - | 2 (100) |
| Oncology (n = 1) | 1 (100) | - | 1 (100) | - | - | 1 (100) |
Table 4. Relationship between the presence of blaVEB, blaGES, and blaPER genes in P. aeruginosa isolates and the type of clinical sample.
| Clinical samples | No. (%) of isolates carrying the relevant gene in the desired clinical samples |
|||||
|
blaVEB
|
blaGES
|
blaPER
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Urine (n = 26) | 7 (26.92) | 19 (73.03) | 6 (23.07) | 20 (76.92) | 7 (26.92) | 19 (73.03) |
| Respiratory (n = 37) | 12 (32.43) | 25 (67.56) | 11 (29.72) | 26 (70.27) | 9 (24.32) | 28 (75.67) |
| Wound (n = 20) | 11 (55) | 9 (45) | 12 (60) | 8 (40) | 12 (60) | 8 (40) |
| Catheter (n = 8) | 7 (87.5) | 1 (12.5) | 5 (62.5) | 3 (37.5) | 4 (50) | 4 (50) |
| Blood (n = 5) | 2 (40) | 3 (60) | 2 (40) | 3 (60) | 2 (40) | 3 (60) |
| Stool (n = 2) | - | 2 (100) | - | 2 (100) | - | 2 (100) |
| Eye (n = 2) | 1 (50) | 1 (50) | - | 2 (100) | 1 (50) | 1 (50) |
4. Discussion
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen and one of the leading causes of nosocomial infections in immunocompromised patients, including patients with a wide range of malignancies, cystic fibrosis, burns, and others [26]. Suitable drugs against infections caused by this bacterium are aminoglycosides, fluoroquinolones, selected β-lactams (e.g., ticarcillin, piperacillin, ceftazidime, aztreonam, and carbapenems), and a β-lactam/β-lactamase inhibitor compound [27]. Treating P. aeruginosa infections has become a major challenge due to the bacterium's ability to develop widespread antibiotic resistance [28]. One of the most prominent features of these bacteria is their resistance to clinically important antibiotics such as third-generation cephalosporins, imipenem, aztreonam, and other important beta-lactams [29].
Acquired resistance to beta-lactams is due to the production of β-lactamase enzymes such as broad-spectrum beta-lactamases (ESBLs), Metallo-β-lactamases (MBLs), and sometimes AmpC plasmid β-lactamases [28]. Beta-lactamases are classified as A, B, C, and D groups by Ambler [12] while sequencing on class A β-lactamase indicated the presence of subclasses A1 and A2 with 100% similarity and highly conserved [30]. The GES enzyme belonged to subgroup A1 of class A ESBLs, is detected in a wide range of countries, and is encoded mainly by plasmids or integrons [30]. These enzymes hydrolyze penicillins, most broad-spectrum cephalosporins, aztreonam, and sometimes carbapenems but not cephamycins, and are inhibited by clavulanate, tazobactam, or imipenem [31],[32]. However, 24 (61.53%), 28 (66.66%), and 27 (69.23%) isolates with resistant or intermediate resistant phenotype to imipenem, meropenem, and doripenem in our study carried the blaGES gene, respectively. This indicates that the presence of this gene may contribute in the development of resistance to carbapenems in our study (P-value = 0.000).
On the other hand, the enzyme blaGES-11 hydrolyzes cefotaxime, ceftazidime, and aztreonam more efficiently than blaGES-1 and is more susceptible to clavulanate and tazobactam [31]. Although 84.61% and 88.88% of the aztreonam and ceftazidime resistant isolates in our study carried the blaGES gene, 91.66% of the isolates that were resistant to piperacillin-tazobactam also carried this gene, indicating that the blaGES-11 enzyme may not have been involved in our study. In a study conducted by an Iranian group on 120 burn P. aeruginosa isolates, 41 isolates were identified as ESBL-producer, while among them, 10 (24.4%) isolates were carrying the blaGES-1 gene [33], while in 2009, a similar study was performed on MDR P. aeruginosa isolates in Tehran and no isolates carried this gene [34]. Increased prevalence of this gene in recent years in Iran and other countries can be a serious warning about the increasing resistance of clinical P. aeruginosa isolates to carbapenems in different parts of the world, while these antibiotics are the last line treatment option before colistin [35]. Another study on 17 P. aeruginosa isolates in a private hospital in Durban, South Africa reported that 13 isolates were ESBL positive, while 10 (58.82%) isolates carried the blaGES-2 gene [36]. This gene hydrolyzes broad-spectrum cephalosporins and imipenem at low levels [36], while 96.29% and 67.74% of the ceftazidime and imipenem resistant isolates in our study carried the blaGES gene. This suggests that other resistance mechanisms may have been involved, or that the gene identified in our study was not blaGES-2. In another study conducted in Hamedan, 43/88 (48.88%) P. aeruginosa isolates were ESBL positive and 2 (2.2%) Isolates carried the blaGES gene [25].
On the other hand, phylogenetic analysis of β-lactamases has shown the presence of genes transmissible through plasmids and integrons such as blaVEB and blaPER in P. aeruginosa [30]. These enzymes are mainly hydrolyzing the cephalosporins, such as cephalothin, ceftazidime, and cefotaxime, as well as aztreonam and penicillins, and fall into class A2 of the Ambler classification [30]. PER-1 and PER-2 are the most common members of the PER family and are less inhibited by avibactam than other class A β-lactamases [18]. However, 93.54% and 64.51% of the isolates resistant to cefepime and imipenem in our study carried the blaPER gene, respectively, while 70.27% and 78.57% of the meropenem and doripenem resistant isolates were carrying this gene, respectively.
VEB-1 enzyme is an effective agent in resistance to ceftazidime, cefotaxime, cefepime, and aztreonam but not imipenem, and can be inhibited by clavulanate [17]. In the present study, 92.3%, 96.29%, and 90.32% of the isolates resistant to aztreonam, ceftazidime, and cefepime carried this gene, indicating the important role of the VEB enzyme in resistance to these antibiotics. Importantly, 67.74%, 72.97%, and 82.14% of the isolates resistant to imipenem, meropenem, and doripenem in our study also carried this gene, but according to the above reference data, other mechanisms or β-lactamase enzymes, including Metallo-β-lactamase, were involved in the development of these resistances. On the other hand, due to the fact that VEB and PER enzymes are inhibited by tazobactam [37], 91.66% of piperacillin-tazobactam resistant isolates in our study also carried this gene, which indicates that the enzyme is not inhibited by tazobactam. Croughs et al., from the Netherlands, reported that out of 1528 P. aeruginosa isolates, 113 were ceftazidime resistant and only 4 (5.3%) isolates had ESBLs, and 2 of these isolates carried the blaVEB-2 gene [38]. Laudy et al., from Poland, reported that out of 900 P. aeruginosa isolates, 99 (11%) cases were ESBL positive, while 69 isolates had blaVEB-9 gene and 14 isolates carrying blaGES genes including blaGES-1 (n = 6), blaGES-5 (n = 1), blaGES-13 (n = 5), and blaGES-15 (n = 2) [39]. In a study conducted in Greece, 29% of the isolates were resistant to ceftazidime, while 1.7% were ESBL positive, and among them, 5 isolates carried the blaPER-1 gene [16]. Also, in a study conducted in 2015 on 200 camel meat samples in Egypt, 45 isolates of P. aeruginosa were obtained, while 21 (46.66%) isolates were identified as ESBL positive and 28.5% of these isolates carried the blaPER-1 gene [24]. This result indicates the importance of transmission of this gene through mobile genetic elements, while the blaPER-1 gene was the most common (55%) class A ESBL gene after the TEM gene (87.5%) in another study on clinical isolates of Acinetobacter baumannii in Egypt [40]. Also, 27.5% of their isolates carried the blaGES gene, but the blaVEB gene was not observed in any of the isolates [40]. Also, in an Egyptian study conducted by Zafer et al. in 2014, 7.4% of the P. aeruginosa isolates were ESBL positive, while 10.4% of them carried the blaVEB-1 gene, and 60.6%, 45.1%, and 25.4% of their isolates were resistant to ceftazidime, aztreonam, and piperacillin-tazobactam, respectively [35].
Moreover, other Iranian studies conducted in 2010 on burn isolates of P. aeruginosa reported that 100% and 31.34% of the isolates contained blaVEB-1 gene, while the blaPER-1 gene was identified in 68.3% and 49.25% of ESBL positive isolates, respectively [33],[41]. Moreover, another study in Hamedan, Iran, showed that their 16 (26.6%) and 9 (15%) ESBL positive isolates contained the blaPER-1 and blaVEB-1 genes [26], while in a study conducted in Ahvaz, the blaPER-1 and blaVEB-1 genes were identified in 2 (6.6%) and 4 (13.3%) ESBL positive isolates, respectively [29].
5. Conclusions
The emergence of antibiotic-resistant strains of Pseudomonas aeruginosa has posed a major challenge in the treatment of infections caused by this bacterium, and resistance to β-lactams as one of the common therapeutic families has also led to treatment deficiencies. On the other hand, the presence of genes encoding ESBLs will increase the rate of problems in the treatment of infections caused by this bacterium. Class A β-lactamases are important in developing resistance to β-lactams. Due to the high ability of the genes encoding these enzymes to move between different organisms through integrons and plasmids, if the rapid spread of these genes is not prevented, none of the gram-negative bacteria will soon respond to any of the β-lactams. This becomes even more important when it causes widespread resistance to carbapenems. Among the most important enzymes in this group of β-lactamases are GES, VEB, and PER enzymes, which over time have increased their prevalence in Iran. Therefore, it is necessary to study the status of antibiotic resistance of Pseudomonas aeruginosa clinical isolates and identify the genes involved in the development of these resistances. However, the limitation of this research was the lack of sequencing of the blaVEB, blaPER, and blaGES genes which hampers the ability to connect back to the AST phenotypes.
Acknowledgments
We thank the laboratory staff of Zare, Razi, Bu-Ali Sina, Fatemeh Zahra, and Imam Khomeini hospitals for providing patients information and the collection of the clinical isolates.
Abbreviations
- MDR
Multi-drug Resistant
- D-Ala-D-Ala
D-Alanine-D-Alanine
- β-lactams
Beta-lactams
- ESBL
Extended-spectrum Beta-lactamase
- OXA
Oxacillinase
- MIC
Minimum Inhibitory Concentration
- TSB
Trypticase Soy Broth
- CLSI
Clinical and Laboratory Standards Institute
- ATCC
American Type Culture Collection
- SDS
Sodium Dodecyl Sulfate
- OD
Optical Density
- PCR
Polymerase Chain Reaction
- ICU
Intensive Care Unit
- CCU
Cardiac Care Unit
- XDR
Extensively Drug Resistant
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Authors' contributions: Hamid R. Goli conceptualized and designed the study protocol development, acquired and analyzed the data. Saboura Haghighi acquired the data, performed all microbiological and molecular laboratory work and drafted the manuscript. All authors revised and approved the final version of the manuscript.
Data Availability: All data generated or analyzed during this study are included in this published article.
Funding: This study is a report of a database from an MSc student thesis registered and carried out in Sana Institute of Higher Education, Sari, Iran, but not funded by any organization.
References
- 1.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agent. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian L, Haghshenas MR, Mirzaei B, et al. Distribution and molecular characterization of resistance gene cassettes containing class 1 integrons in multi-drug resistant (MDR) clinical isolates of Pseudomonas aeruginosa. Infect Drug Resist. 2020;13:2773. doi: 10.2147/IDR.S263759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klockgether J, Cramer N, Wiehlmann L, et al. Pseudomonas aeruginosa genomic structure and diversity. Front Microbiol. 2011;2:150. doi: 10.3389/fmicb.2011.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microb Infect. 2000;2:1051–1060. doi: 10.1016/S1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E, Magrini N, Kahlmeter G, et al. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organiz. 2017;27:318–327. Available from: https://www.aidsdatahub.org/resource/who-global-priority-list-antibiotic-resistant-bacteria. [Google Scholar]
- 7.Pang Z, Raudonis R, Glick BR, et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotech Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Thakuria B, Lahon K. The beta lactam antibiotics as an empirical therapy in a developing country: an update on their current status and recommendations to counter the resistance against them. J Clin Diagn Res JCDR. 2013;7:1207. doi: 10.7860/JCDR/2013/5239.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim ME, Abbas M, Al-Shahrai AM, et al. Phenotypic characterization and antibiotic resistance patterns of extended-spectrum β-Lactamase-and AmpC β-lactamase-producing Gram-negative bacteria in a referral hospital, Saudi Arabia. Canadian J Infect Dis Med Microbiol. 2019;2019 doi: 10.1155/2019/6054694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasser M, Gayen S, Kharat AS. Prevalence of β-lactamase and antibiotic-resistant Pseudomonas aeruginosa in the Arab region. J Glob Antimicrob Resist. 2020;22:152–160. doi: 10.1016/j.jgar.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Matlashewski G, Berghuis A, Sheppard D, et al. Handbook of Antimicrobial Resistance: Springer. 2017. ISBN: 978-1-4939-0694-9.
- 12.Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 13.Papp-Wallace KM, Becka SA, Taracila MA, et al. Exposing a β-lactamase “twist”: the mechanistic basis for the high level of ceftazidime resistance in the C69F variant of the Burkholderia pseudomallei PenI β-lactamase. Antimicrob Agents chemother. 2016;60:777–788. doi: 10.1128/AAC.02073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossolini G, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin Microbiol Infect. 2005;11:17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 15.Bauernfeind A, Stemplinger I, Jungwirth R, et al. Characterization of beta-lactamase gene blaPER-2, which encodes an extended-spectrum class A beta-lactamase. Antimicrob Agents chemother. 1996;40:616–620. doi: 10.1128/AAC.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranellou K, Kadlec K, Poulou A, et al. Detection of Pseudomonas aeruginosa isolates of the international clonal complex 11 carrying the blaPER-1 extended-spectrum β-lactamase gene in Greece. J Antimicrob Chemother. 2012;67:357–361. doi: 10.1093/jac/dkr471. [DOI] [PubMed] [Google Scholar]
- 17.Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime+ NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010;65:2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz de la Rosa JM, Nordmann P, Poirel L. ESBLs and resistance to ceftazidime/avibactam and ceftolozane/tazobactam combinations in Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother. 2019;74:1934–1939. doi: 10.1093/jac/dkz149. [DOI] [PubMed] [Google Scholar]
- 19.Castanheira M, Simner PJ, Bradford PA. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC-Antimicrob Resist. 2021;3:dlab092. doi: 10.1093/jacamr/dlab092. https://doi.org/10.1093/jacamr/dlab092. https://doi.org/10.1093/jacamr/dlab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahm DF, Weissfeld A, Trevino E. Baily and Scott's diagnostic microbiology. Mosby, St Louis. 2002 ISBN: 9780323681056. [Google Scholar]
- 21.Wayne P. CLSI document M100-S30. Maryland, USA: Clinical and Laboratory Standards Institute; 2020. Performance standards for antimicrobial susceptibility testing; 29th informational supplement. ISBN: 978-168440-067-6. [Google Scholar]
- 22.Šipošová NŠ, Liptáková V, Kvasnová S, et al. Genetic diversity of Acinetobacter spp. adapted to heavy metal polluted environments. Nova Biotech et Chim. 2017;16:42–47. doi: 10.1515/nbec-2017-0006. [DOI] [Google Scholar]
- 23.Jiang X, Zhang Z, Li M, et al. Detection of extended-spectrum β-lactamases in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2990–2995. doi: 10.1128/AAC.01511-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhariri M, Hamza D, Elhelw R, et al. Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in Egypt: potential human hazard. Ann Clin Microbiol Antimicrob. 2017;16:1–6. doi: 10.1186/s12941-017-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehbashi S, Tahmasebi H, Alikhani MY, et al. Distribution of Class B and Class A β-lactamases in clinical strains of Pseudomonas aeruginosa: comparison of phenotypic methods and high-resolution melting analysis (HRMA) assay. Infect Drug Resist. 2020;13:2037. doi: 10.2147/IDR.S255292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alikhani MY, Tabar ZK, Mihani F, et al. Antimicrobial resistance patterns and prevalence of blaPER-1 and blaVEB-1 genes among ESBL-producing Pseudomonas aeruginosa isolates in West of Iran. Jundishapur J Microbiol. 2014;7:e8888. doi: 10.5812/jjm.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endimiani A, Luzzaro F, Pini B, et al. Pseudomonas aeruginosa bloodstream infections: risk factors and treatment outcome related to expression of the PER-1 extended-spectrum beta-lactamase. BMC Infect Dis. 2006;6:1–9. doi: 10.1186/1471-2334-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabiei MM, Asadi K, Shokouhi S, et al. Antipseudomonal β-Lactams Resistance in Iran. Int J Microbiol. 2020;2020:8818315. doi: 10.1155/2020/8818315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokaeian M, Zahedani SS, Bajgiran MS, et al. Frequency of PER, VEB, SHV, TEM and CTX-M genes in resistant strains of Pseudomonas aeruginosa producing extended spectrum β-lactamases. Jundishapur J Microbiol. 2015;8:e13783. doi: 10.5812/jjm.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philippon A, Slama P, Dény P, et al. A structure-based classification of class A β-lactamases, a broadly diverse family of enzymes. Clin Microbiol Rev. 2016;29:29–57. doi: 10.1128/CMR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delbrück H, Bogaerts P, Kupper MB, et al. Kinetic and crystallographic studies of extended-spectrum GES-11, GES-12, and GES-14 β-lactamases. Antimicrob Agents Chemother. 2012;56:5618–5625. doi: 10.1128/AAC.01272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naas T, Poirel L, Nordmann P. Minor extended-spectrum β-lactamases. Clin Microbiol Infect. 2008;14:42–52. doi: 10.1111/j.1469-0691.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 33.Shacheraghi F, Shakibaie MR, Noveiri H, et al. Molecular Identification of ESBL Genes blaGES-1, blaVEB-1, blaCTX-M, blaOXA-1, blaOXA-4, blaOXA-10 and blaPER-1 in Pseudomonas aeruginosa Strains Isolated from Burn Patients by PCR, RFLP and Sequencing Techniques. Int J Biol Life Sci. 2010;6:138–142. doi: 10.5281/zenodo.1327919. [DOI] [Google Scholar]
- 34.Shahcheraghi F, Nikbin VS, Feizabadi MM. Prevalence of ESBLs genes among multidrug-resistant isolates of Pseudomonas aeruginosa isolated from patients in Tehran. Microb Drug Resist. 2009;15:37–39. doi: 10.1089/mdr.2009.0880. [DOI] [PubMed] [Google Scholar]
- 35.Zafer MM, Al-Agamy MH, El-Mahallawy HA, et al. Antimicrobial resistance pattern and their beta-lactamase encoding genes among Pseudomonas aeruginosa strains isolated from cancer patients. BioMed Res Int. 2014;2014:101635. doi: 10.1155/2014/101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adjei CB, Govinden U, Essack SY, et al. Molecular characterisation of multidrug-resistant Pseudomonas aeruginosa from a private hospital in Durban, South Africa. South African J Infect Dis. 2018;33:38–41. doi: 10.4102/sajid.v33i2.19. [DOI] [Google Scholar]
- 37.Sawa T, Kooguchi K, Moriyama K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J Intensive Care. 2020;8:1–13. doi: 10.1186/s40560-020-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croughs P, Klaassen C, van Rosmalen J, et al. Unexpected mechanisms of resistance in Dutch Pseudomonas aeruginosa isolates collected during 14 years of surveillance. Int J Antimicrob Agents. 2018;52:407–410. doi: 10.1016/j.ijantimicag.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Laudy AE, Róg P, Smolińska-Król K, et al. Prevalence of ESBL-producing Pseudomonas aeruginosa isolates in Warsaw, Poland, detected by various phenotypic and genotypic methods. PloS one. 2017;12:e0180121. doi: 10.1371/journal.pone.0180121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Agamy MH, Khalaf NG, Tawfick MM, et al. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis. 2014;22:49–54. doi: 10.1016/j.ijid.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Mirsalehian A, Feizabadi M, Nakhjavani FA, et al. Detection of VEB-1, OXA-10 and PER-1 genotypes in extended-spectrum β-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients. Burns. 2010;36:70–74. doi: 10.1016/j.burns.2009.01.015. [DOI] [PubMed] [Google Scholar]