Abstract
New hybrid liposomes based on cationic amphiphiles with different structures of the head group (cetyltrimethylammonium bromide (CTAB), 3-hexadecyl-1-hydroxyethylimidazolium bromide (IA-16(OH)), 1-(butylcarbamoyl)oxyethyl-3-hexadecylimidazolium bromide (IAC 16(Bu)), and hexadecylmethylpyrrolidinium bromide (PR-16)) were developed for transdermal administration of nonsteroidal anti-inflammatory drugs. The different surfactant/lipid compositions were studied to obtain stable liposomes with high functionality. The hydrodynamic diameter of cationic liposomes was ∼110 nm. An admixture of cationic surfactants and PC liposomes improves the physicochemical properties of vesicles and transdermal diffusion rate and prolongs the release of drugs. Liposomal diclofenac sodium (DS) and ketoprofen (KP) were tested (using Franz cells) for transdermal penetration. Drug diffusion monitoring for 48 h demonstrated that the maximum DS and KP penetration through the synthetic membranes (Strat-M) is characterized by values of 255 ± 2 and 186 ± 3 μg/cm2, respectively. The influence of the surfactant head group on the properties (stability, release profile, permeability) of cationic liposomes was shown for the first time. While the drug specificity is evident for the rate of release, the permeability increases as follows: conventional liposomes < CTAB/PC < PR-16/PC < IAC-16(Bu)/PC < IA-16(OH)/PC for both medicines. The rat paw edema model was used to assess the anti-inflammatory effect of the IA-16(OH)/PC leader formulation in vivo. It was found that liposomal DS and KP are effective for relieving rat paw edema. It should be noted that DS-loaded hybrid liposomes demonstrated the highest therapeutic efficacy compared to conventional vesicles.
1. Introduction
Among the numerous routes of administration, transdermal drug delivery (TD) holds an essential place, as it allows for the direct access of active compounds through the skin surface to the bloodstream, hepatic avoidance, noninvasiveness, self-administration, and high patient compliance.1 In addition, TD can improve drug bioavailability, maintain plasma levels due to slow release, and provide relief from gastrointestinal disturbances. The efficiency of the transdermal drug penetration is strongly dependent on the skin barrier function, mainly, the stratum corneum (SC). Despite numerous investigations of transdermal systems, the low SC permeability limits the range of drugs that are suitable for topical and transdermal routes of administration.2 Meanwhile, it has recently been documented that lipid vesicles are actively used to overcome the SC.3,4
The interaction of liposomes with the skin and further transdermal drug diffusion depend on several factors conjugated with the nature of loads and features of nanocarriers and structural conditions occurring inside the skin and bioenvironment.5 Among others, an essential factor is the physical state of the lipid bilayer. The phospholipid bilayers in the liquid crystalline state are beneficial for transdermal medicine delivery, providing a closer contact with the drug formulation. Based on this criterion, liposomal nanocarriers can be grouped into conventional and new deformable/flexible/elastic liposomes.6 Conventional liposomes are believed to be not suitable for transdermal drug delivery. Their rigid structure does not allow penetration into the deep skin layers, and therefore, unmodified liposomal formulations were retained in the corneous layer.7−9 Moreover, the use of conventional liposomes is limited due to their insufficient stability and low encapsulation efficiency and loading capacity.10−13 Hence, a variety of strategies are developed for the design of a new generation of liposomal formulations with improved characteristics. One of the ways focuses on the above-mentioned deformable liposomal formulations with improved permeability, such as transfersomes,14 ethosomes,15 invasomes,16 mentosomes,17 and niosomes.18 The majority of these vehicles involve an edge activator that can endow them with the required elasticity. Also, it can destabilize the lipid membrane and increase its fluidity due to the redistribution of the lipids.19,20 Usually, a surfactant with a large radius of curvature acts as an edge activator.21−24
One more finding is that the transdermal route of administration can also depend on changing the charge of liposomes. However, there is no consensus on this issue. The lipid bilayer of the corneous layer contains a large number of negatively charged fragments.25 Thus, positively charged nanocontainers can better penetrate through the skin.26 Ref (27) demonstrated that transdermal penetration of liposomal amphotericin B was better upon using charged liposomes compared with uncharged ones. Moreover, vesicles with a positive charge passed through the SC more effective than negative ones. In ref (28), authors prepared cationic transfersomes loaded with meloxicam. The introduction of cationic surfactants into the formulations allows us not only to improve the physicochemical characteristics of nanocontainers but also to provide greater penetration of the drug through the skin, compared to unmodified vesicles and suspensions. Also, the authors found that increased permeation of cationic transfersomes through the skin occurs due to the mechanisms of vesicle adsorption and fusion with the SC.28 These authors also established meloxicam-loaded transfersomes modified with anionic surfactants. It was shown that these transfersomes ensured greater permeation of meloxicam through the skin compared to liposomes and suspensions. However, the particle penetration mechanism differed from that of cationic transfersomes. The authors found that the increase in the penetration of meloxicam through the skin is due to the destruction of SC lipids by transfersomes.29 In refs30, 31, better penetration of negatively charged nanocontainers was shown. Thus, the delivery mechanisms of different liposomal nanocontainers through the skin are not well understood. The mechanism can change the properties of the liposomal formulation and medicine.32 Therefore, the search for new penetration enhancers for transdermal drug delivery is a significant area of research.
Recently, our study has been published, demonstrating successful transdermal drug diffusion in vitro, ex vivo, and in vivo with the use of ketoprofen-loaded liposomes noncovalently modified with cationic surfactants bearing a hydroxyethyl-substituted pyrrolidinium head group.33 To confirm this trend and elucidate the effects of the structure of the surfactant and drug, we launch novel work involving cationic liposomes modified with surfactants with different structures of head groups and a hexadecyl tail. According to the literature data, there are a few studies on the effect of cationic surfactant head groups in the liposome composition on the ability for transdermal drug delivery. Cationic surfactants are good modifiers due to their positive charge, which can help to improve drug penetration through the SC. Moreover, cationic surfactants may act as an edge activator due to their ability to integrate into the lipid bilayer and promote the liquid crystalline behavior of the lipid bilayer.34−36 Therefore, in this study, hybrid liposomes loaded with the probe Rhodamine B (RhB) and nonsteroidal anti-inflammatory drugs—diclofenac sodium (DS) and ketoprofen (KP)—were obtained. A variety of cationic surfactants with a hexadecyl tail and different head groups were studied. Along with typical cationic surfactant cetyltrimethylammonium bromide (CTAB), amphiphilic compounds with cationic head groups have been chosen, including unsubstituted analogues of the hydroxypyrrolidinium surfactant used in ref (30) (PR-16) and imidazolium derivatives bearing hydroxyethyl (IA-16(OH)) and carbamate (IAC-16(Bu)) fragments. For these cationic surfactants, self-assembling behavior and biomedical potential have been earlier evaluated.37−39 These data are used herein as basic fundamental information. In the present work, for all the liposomal formulations, particle size, ζ potential, stability over the time, and substrate release rate were evaluated. Liposomal DS and KP were tested for the ability of transdermal diffusion using Franz cells and were investigated for the ability to relieve the rats’ paw inflammation caused by carrageenan injection. The chemical structures of the compounds used in the work are shown in Figure 1.
Figure 1.
Chemical structure of the compounds used in the work.
2. Materials and Methods
2.1. Materials
Cetyltrimethylammonium bromide (CTAB) (Sigma-Aldrich, ≥98%), 3-hexadecyl-1-hydroxyethylimidazolium bromide (IA-16(OH)), 1-[2-(butylcarbamoyl)oxyethyl]-3-hexadecyl-1H-imidazol-3-ium bromide (IAC 16(Bu)), and 1-hexadecyl-1-methylpyrrolidinium bromide (PR-16) were synthesized by previously described procedures.38−40 Lipoid S PC (98%) was gifted from Lipoid GmbH (Ludwigshafen, Germany). Rhodamine B (Sigma-Aldrich, ≥95%), ketoprofen (Sigma, ≥98%), and diclofenac sodium (Sigma, ≥99%) were used without prior purification.
2.2. Preparation of Liposomes
The preparation of cationic vesicles was carried out by the method of hydration of the lipid film.41 In brief, PC powder was mixed with the desired amount of surfactant in molar ratios of surfactant/lipid components of 0.02/1 (0.4 mM surfactant/20 mM lipid), 0.029/1 (0.57 mM surfactant/20 mM lipid), and 0.04/1 (0.8 mM surfactant/20 mM lipid). The mixture was dissolved in an organic solvent (chloroform 100–200 μL). After complete removal of the solvent, the formed lipid film was hydrated and stirred at 55–60 °C for 30 min. Next, the freeze/thaw procedure was performed five times. Furthermore, the liposome solution was passed through a LiposoFast Basic extruder 20 times using Whatman Nuclepore track-etched membranes (100 nm pore size).
2.3. Loading of the Drugs into Liposomes
Fabrication of drug-loaded vesicles was carried out using the manipulations described in the Section 2.2. An aqueous solution of the RhB (0.5 mg/mL) and DS (5 mg/mL) was added to the lipid film. In the case of hydrophobic KP (0.7 mg/mL), surfactants, lipid, and KP were mixed in a dry consistency in certain quantities. The mixture was dissolved in an organic solvent (chloroform 100–200 μL), dried, and dispersed with water.
2.4. Size and ζ Potential of Hybrid Liposomes
The hydrodynamic radius (RH) and ζ potential of hybrid liposomes were estimated using dynamic and electrophoretic light scattering (DLS) on a ZetaSizer Nano apparatus (Malvern, UK).42,43 The autocorrelation functions were analyzed using Malvern DTS software, using intensity-weighted distribution functions calculated by inverse Laplace transformation and Z-averaged values of hydrodynamic radius and polydispersity index (PdI) by applying the cumulant expansion method. The Stokes–Einstein equation was used for the calculation of RH
| 1 |
where T is the absolute temperature, D is the translational diffusion coefficient, η is the solvent viscosity, and k is Boltzmann’s constant. The solutions were diluted to 1 mM and measured five times.44 The ζ potential was calculated using the Smoluchowski equation
| 2 |
where ζ is the zeta potential, η is the dynamic viscosity of a solvent, μ is the particle electrophoretic mobility, and ε is the dielectric constant.45 Electrophoretic mobility for all samples is given in the Supporting Information (Tables S1–S2, Figure S1).
2.5. Transmission Electron Microscopy
Liposomes for transmission electron microscopy (TEM) were prepared according to the same procedure as described in the Section 2.2. TEM images were obtained in the Interdisciplinary Center for Analytical Microscopy of KFU, using a Hitachi HT7700 Exalens microscope (Japan).46,47 The images were acquired at an accelerating voltage of 100 keV in the high-contrast mode. A sample of 3 μL was dispersed on 300-mesh 3 mm copper grids (Ted Pella) with continuous carbon Formvar support films. The samples were dried at room temperature under normal conditions.
2.6. In Vitro Drug Release Studies and Quantitative Parameter of Encapsulation
The in vitro release study of substrates (RhB, DS, or KP) from hybrid vesicles was performed using a Specord 250 Plus (Analytik Jena, Germany). The release of substrates was monitored using the dialysis bag (3.5 kDa molecular weight cutoff) diffusion method. At certain intervals, 2 ml aliquots of the sample were taken from the external medium. The content of substrates in the samples was determined by measuring the optical density at a wavelength of 555 nm for RhB, 275 nm for DS, and 260 nm for CP. The spectrophotometric data were used to plot the substrate release profile, in which the solid lines are given to guide the eye. Further calculations were performed using the Bouguer–Lambert–Beer law (extinction coefficient for RhB was taken as 94 000 M−1·cm−1, with 14 957 M−1·cm−1 for DS (Figure S2a) and 23 477 M−1·cm−1 for KP (Figure S2b)).
Encapsulation efficiency was determined by spectrophotometry. The proportion of the unencapsulated substrate was found using centrifuge concentrators: the liposome samples was centrifuged (10 min at 10 000 rpm) and then, the concentration of substances was determined.
| 3 |
2.7. In Vitro Transdermal Diffusion Study
The vertical Franz cells were used to study the transdermal penetration of substrates in vitro. All experiments were performed under the same conditions: 32 ± 1 °C,48 500 rpm, diffusion area of 0.785 cm2, phosphate buffer (PB) (pH = 7.4, 0.025 M) volume in the capacity receptor of 5 mL, and sample volume in the donor cell of 400 μL. Synthetic membranes made of polyethersulfone Strat-M (Merck Millipore, diameter of 25 mm) acted as a skin model. A sample was manually taken from the receptor compartment and replaced with fresh PB at each time point. The amount of the substrate in the sample was estimated spectrophotometrically (Specord 250 Plus).
2.8. Assay of the Anti-inflammatory Activity of Liposomal Formulations
The experiments with rats were carried out according to the European Union Council Directive 2010/63/EU and the protocol approved by the Animal Care and Use Committee of Kazan Federal University. The Wistar rats (males) weighing 200–300 g were purchased from the Animal Breeding Facility of the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry (Puschino, Russia). Before the experiments, the animals were acclimatized to the environment for at least 1 week. Animals were kept in plastic cages with sawdust, 12 h light/dark cycles, 20–22 °C, a humidity of 60–70%, and ad libitum access to food and water.
Acute inflammation was studied using a rat paw edema model induced by injection of carrageenan as previously described.33,49 The volume of the paw was measured using a plethysmometer (Ugo Basile, Varese, Italy). The mesuments were performed before the injection of carrageenan and 1, 2, 3, 4, 5 and 24 h after the injection of carrageenan. A new bandage with the liposomal formulations was wrapped after each measurement. The rats were randomly divided into groups, containing six animals in each group. The rats of the control group were treated with physiological saline. The experimental groups of animals were treated with the test compositions.
All data are given as means ± SE. Statistical significance was estimated using one way analysis of variance (ANOVA) followed by a post hoc test at the level of p < 0.05.
3. Results and Discussion
3.1. Fabrication and Physiochemical Properties of Empty Hybrid Liposomes
Many parameters of the vesicles (size, charge, stability over time, encapsulation efficiency, drug release rate) depend on the vesicle composition. Therefore, at the first stage, the composition of the empty hybrid lipid formulations was optimized by varying the surfactant/PC ratios (0.02/1, 0.029/1, 0.04/1). The charge, size, and stability of liposomes over time were measured by dynamic and electrophoretic light scattering (Figures 2–3, Table S3).
Figure 2.
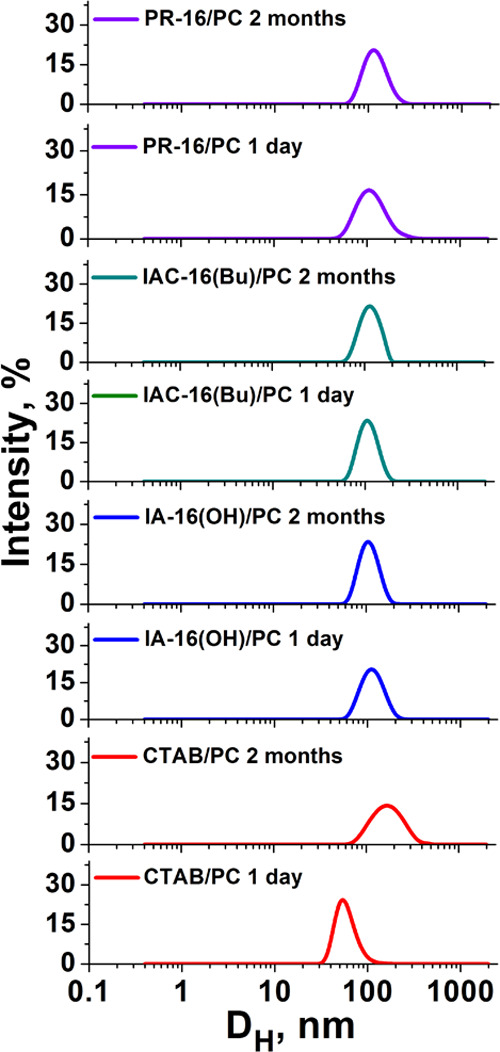
Intensity–size distribution for the surfactant/PC hybrid vesicles with a molar ratio of 0.029/1; 25 °C.
Figure 3.
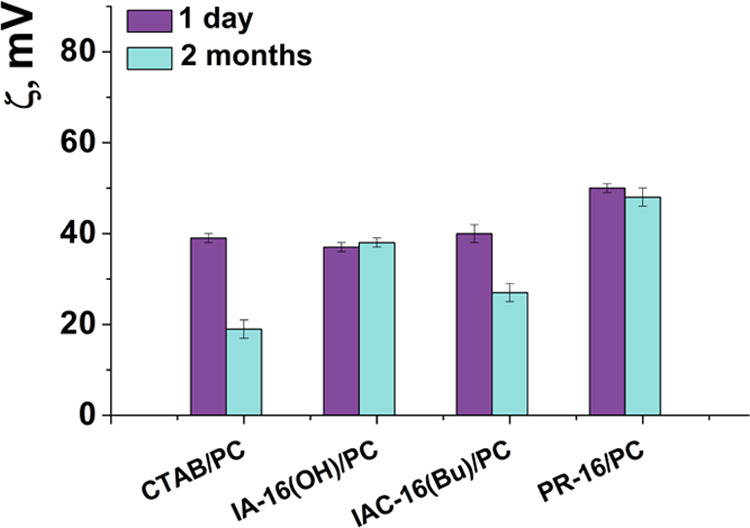
Electrokinetic potential of surfactant/PC hybrid vesicles with a molar ratio of 0.029/1; 25 °C.
Liposome size is the key parameter, which determines the effectiveness of the transdermal drug penetration. According to refs50, 51, the aggregates with a size of ∼80–120 nm are optimal for transdermal diffusion. In our study, the Dh of the liposomes varies from 84 to 114 nm according to DLS data. The polydispersity index(PdI) of liposomes is lower than 0.2, which indicates a narrow size distribution of aggregates (Table S3). The ζ potential of the vesicles ranges from +28 to +54 mV and depends on the composition of the liposomes. For the studied systems, an increase in the amount of surfactants in the composition of liposomes leads to an increase in the ζ potential of liposomes. This is probably due to the high content of amphiphile molecules in the lipid bilayer. In addition, decorating liposomes with cationic surfactants improves the stability of the systems. In particular, hybrid liposomes are stable for at least 2 months; the stability of PC liposomes does not exceed 2 weeks. Worth noting is that there are no significant changes in the physicochemical parameters of liposomes over the storage time.
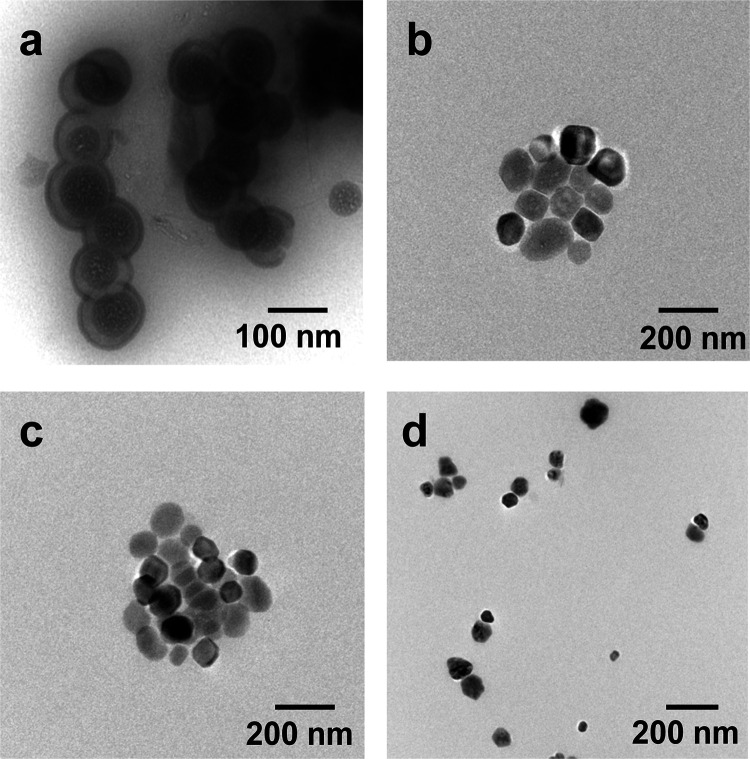
The morphology of the liposomes (surfactant/PC molar ratio of 0.029/1) was studied by transmission electron microscopy. The TEM image shows a good correlation with the DLS data (Figure 4). Liposomes are mostly monodisperse and have a spherical shape.
Figure 4.
TEM images of the surfactant/PC hybrid liposomes with a molar ratio of 0.029/1 (on the first day after preparation of liposomes): (a) CTAB/PC; (b) IA-16(OH)/PC; (c) IAC-16(Bu)/PC; and (d) PR-16/PC; 25 °C.
3.2. Preparation of the Rhodamine B-Loaded Cationic Hybrid Liposomes and In Vitro Release Study
A hydrophilic probe, RhB, was loaded into hybrid liposomes, and the physicochemical characteristics were assessed. The loading of the probe into liposomes insignificantly affects the particle size, but it changes the ζ potential (Tables 1 and S4). The ζ potential of RhB-loaded liposomes is higher than that of empty vesicles. This is probably due to the existence of the probe as a salt. A high positive charge ensures the stability of liposomes for a long time (more than 2 months). An increase in PdI indexes occurring for some compositions upon the storage probably reflects the partial degradation of samples accompanied by changes in the ζ potential. EE was calculated for all systems for evaluation of the amount of the substrate successfully encapsulated into liposomes. The EE was essentially independent of the component’s molar ratio, but it changes when varying the surfactant head group. In particular, the highest EE values are observed for CTAB/PC and PR-16/PC systems (89–92%), with lower values observed for IA-16(OH)/PC and IAC-16(Bu)/PC systems (69–80%) (Tables 1 and S4).
Table 1. Size (DH = 2RH, nm), ζ Potential (ζ, mV), Polydispersity Index (PdI), and Encapsulation Efficiency EE (%) of Substrate-Loaded Hybrid Surfactant–PC Liposomes at a Molar Ratio of 0.029/1, 25 °C.
| 1 day |
2 months |
||||||
|---|---|---|---|---|---|---|---|
| system | EE, % | DH, nm | PdI | ζ, mV | DH, nm | PdI | ζ, mV |
| Rhodamine B | |||||||
| PC | 34 ± 1 | 105 ± 2 | 0.055 ± 0.002 | –3 ± 1 | not stable | ||
| CTAB/PC | 92 ± 1 | 122 ± 2 | 0.088 ± 0.015 | 57 ± 1 | 141 ± 1 | 0.219 ± 0.010 | 50 ± 2 |
| IA-16(OH)/PC | 81 ± 1 | 111 ± 1 | 0.089 ± 0.006 | 53 ± 1 | 122 ± 2 | 0.096 ± 0.016 | 47 ± 1 |
| IAC-16(Bu)/PC | 69 ± 1 | 109 ± 2 | 0.078 ± 0.002 | 47 ± 1 | 112 ± 2 | 0.074 ± 0.003 | 39 ± 1 |
| PR-16/PC | 90 ± 1 | 123 ± 2 | 0.172 ± 0.010 | 51 ± 1 | 125 ± 1 | 0.174 ± 0.066 | 50 ± 2 |
| Diclofenac Sodium | |||||||
| PC | 50 ± 2 | 99 ± 4 | 0.115 ± 0.035 | –23 ± 1 | 86 ± 4 | 0.250 ± 0.085 | –10 ± 2 |
| CTAB/PC | 82 ± 1 | 127 ± 1 | 0.114 ± 0.017 | –53 ± 1 | 119 ± 3 | 0.116 ± 0.015 | –44 ± 4 |
| IA-16(OH)/PC | 80 ± 1 | 104 ± 1 | 0.049 ± 0.015 | –37 ± 1 | 113 ± 3 | 0.056 ± 0.009 | –31 ± 2 |
| IAC-16(Bu)/PC | 78 ± 1 | 100 ± 1 | 0.100 ± 0.018 | –38 ± 2 | 103 ± 2 | 0.121 ± 0.017 | –29 ± 4 |
| PR-16/PC | 81 ± 1 | 111 ± 2 | 0.053 ± 0.017 | –42 ± 1 | 115 ± 3 | 0.053 ± 0.010 | –35 ± 2 |
| Ketoprofen | |||||||
| PC | 98 ± 1 | 92 ± 2 | 0.091 ± 0.002 | –7 ± 1 | not stable | ||
| CTAB/PC | 99 ± 1 | 104 ± 2 | 0.128 ± 0.002 | 34 ± 1 | 112 ± 2 | 0.176 ± 0.012 | 15 ± 1 |
| IA-16(OH)/PC | 98 ± 1 | 101 ± 2 | 0.106 ± 0.012 | 32 ± 1 | 105 ± 2 | 0.080 ± 0.012 | 19 ± 1 |
| IAC-16(Bu)/PC | 98 ± 1 | 126 ± 2 | 0.176 ± 0.016 | 30 ± 1 | 122 ± 1 | 0.181 ± 0.012 | 12 ± 1 |
| PR-16/PC | 99 ± 1 | 106 ± 2 | 0.095 ± 0.014 | 36 ± 1 | 116 ± 2 | 0.187 ± 0.020 | 17 ± 1 |
In vitro RhB release study was carried out by the dialysis technique using a spectrophotometric method. Absorption spectra were recorded at regular intervals (Figures S3-S6). The spectrophotometric data were used to plot the substrate release dependence on time (Figures 5 and S7). Figure 5 shows that 93% release of RhB from PC liposomes occurs for about 8 h; its encapsulation in hybrid vesicles leads to a slower release increase in the release time (Figure S7). From the IA-16(OH)/PC and IAC-16(Bu)/PC systems for 8 h, about 80% of the substrate was released. For CTAB/PC and PR-16/PC liposomes, 58 and 70% of RhB are released during this time, respectively (Figure 5). This may be because the imidazolium surfactants increasingly loosen the structure of the liposomes, which leads to a faster release. It is noteworthy that the RhB release rate is practically independent of the surfactant/lipid molar ratio.
Figure 5.
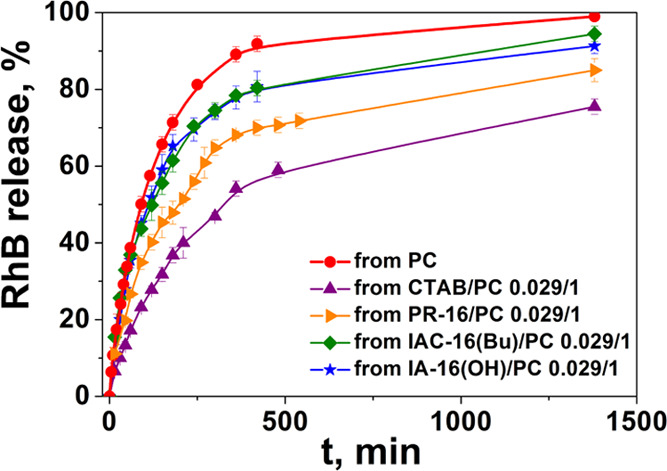
In vitro release profile of the RhB-loaded surfactant/PC liposomes at a molar ratio of 0.029/1: CTAB/PC; IA-16(OH)/PC; IAC-16(Bu)/PC; PR-16/PC, PB (25 mM), pH 7.4, 37 °C.
3.3. Drug-Loaded Hybrid Liposomes
DS is a nonsteroidal anti-inflammatory drug (NSAID) widely used for the treatment of rheumatoid arthritis, joint disease, and ankylosing spondylitis.52 Also, it is applied to relieve the pain resulting from minor surgery, trauma, and dysmenorrhea.52−54 However, oral application of DS can lead to stomach discomfort and the risk of inducing ulcers. Transdermal delivery of DS allows for avoiding first-pass metabolism and eliminating the gastrointestinal side effects.55,56 Therefore, hybrid liposomes have been evaluated as potential systems for the transdermal delivery of DS.
The DH, ζ, and PdI of the drug-loaded vesicles were analyzed using DLS (Tables 1 and S4). In contrary with RhB, encapsulation of DS into liposomes did not affect the hydrodynamic diameter of liposomes. The PdI was below 0.2, indicating the monodispersity of the systems. There is an interesting trend for the ζ potential of particles: encapsulation of DS changes the positive charge of the cationic liposome to negative. This may be due to the adsorption of negatively charged DS ions on the liposomes. Moreover, the higher the ζ potential in empty liposomes was, the more the negative charge in DS-loaded liposomes was observed, thereby supporting the electrostatic mechanism of the effect observed. It should be taken into account that the additional adsorption of ions on the surface of liposomes can increase the EE. For PC liposomes, the EE value did not exceed 50%, while for the hybrid liposomes, the EE was about 80–85% (Tables 1 and S4).
The next step was the fabrication of KP-loaded hybrid liposomes. As mentioned earlier, KP is a NSAID, which is widely used to treat musculoskeletal disorders.57 DLS data testify that the DH of vesicles is about 100–120 nm, with PdI not exceeding 0.2 (Tables 1 and S4). The ζ potential of the KP-loaded hybrid liposomes ranges from +26 to +42 mV depending on the ratio of the PC/surfactant (Tables 1 and S4). For the studied systems, the increase in the concentration of the surfactant leads to the increase in the ζ potential. It should be noted that the ζ potential of the KP-loaded liposomes is lower than that of the empty vesicles. Liposomes were stable for more than two months. However, a gradual reduction of the charge and an increase of the polydispersity of the systems were observed over time. The KP-loaded liposomes are characterized by an excellent encapsulation efficiency of 97–98%. This is probably due to the hydrophobic nature of the drug, which allows it to be completely distributed in the lipid membrane of the vesicles.
3.4. In Vitro Release Profile
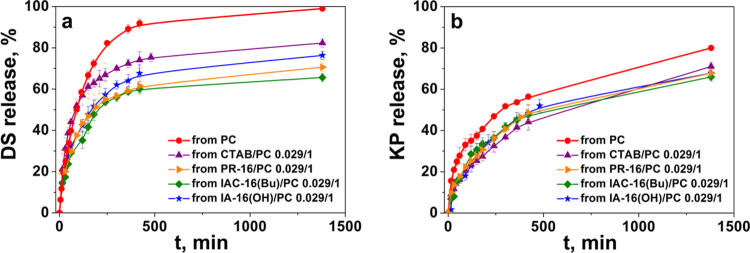
The release profiles of DS and KP were assessed using the dialysis technique by UV–vis spectroscopy (Figures S8–S11, S13–S16). The DS release rate estimation showed that the drug release is essentially independent of the surfactant-to-PC ratio, similar to RhB. It has been shown that the embedding of the surfactant into the bilayer of the PC promotes the prolonged release of DS in contrast to conventional liposomes (Figure S12). The DS yield is 92% from unmodified liposomes and only 70% from hybrid liposomes for 7 h. Depending on the surfactant head group, the DS release rate decreases as follows: CTAB/PC > IA-16(OH)/PC > PR-16/PC > IAC-16(Bu)/PC (Figure 6a).
Figure 6.
In vitro DS (a) and KP (b) release from mixed liposomes at a surfactant/PC ratio of 0.029/1: CTAB/PC; IA-16(OH)/PC; IAC-16(Bu)/PC; PR-16/PC, PB (25 mM), pH 7.4, 37 °C.
The specificity of KP was shown. While prolonged KP release is observed for cationic hybrid liposomes compared to unmodified liposomes, their release rate differs to a less extent than in the case of DS formulations (Figures 6b and S17). In particular ∼55 ± 4% of the substrate is released from the PC liposomes, while 43–50% release occurred from the hybrid liposomes within 8 h. In addition, the surfactant structure and the molar ratio of the components have little effect on the rate of KP release. (Figures 6b and S17). This is probably due to the physicochemical properties of KP. It is poorly soluble in water and therefore located in the liposome lipid bilayer. Likely, the release rate is mainly due to the concentration gradient between the particles and the environment.
3.5. Transdermal Penetration Study and Anti-inflammatory Activity of Drug Liposomal Formulations
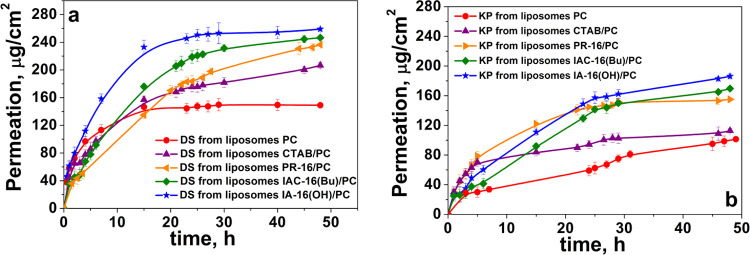
Substrate permeation study through different barriers (synthetic or natural) is a commonly used method for determining the transdermal delivery system efficiency. Franz diffusion cells are often used to demonstrate the transdermal penetration. According to ref (58), in vitro permeation study of DS- and KP-loaded hybrid liposomes was carried out at a temperature of 32 ± 1 °C using Strat-M membranes.59−61 The DS and KP penetration profiles are shown in Figure 7.
Figure 7.
In vitro permeation of DS (a) and KP (b) (μg/cm2) through the Strat-M membrane for 48 h from hybrid and conventional liposomes, PB (25 mM), pH = 7.4, 32 ± 1 °C.
The membranes are composed of 300 μm-thick polyethersulfone and showed a good agreement in the permeability data (compared with human skin), which allows it to be used as a surrogate to human skin.59−61
Conventional liposomes are unsuitable for the transdermal administration of drugs because they can be retained in the SC.62,63 Therefore, DS- and KP-loaded PC liposomes have predictably shown low permeation through the Strat-M membrane. Modification of liposomes with cationic surfactants can significantly enhance the transdermal drug penetration. Moreover, the surfactant head group structure strongly affects the penetration properties of liposomes through the Strat-M. For DS, this ability is enhanced as follows: PC < CTAB/PC < PR-16/PC < IAC-16(Bu)/PC < IA-16(OH)/PC. The total DS that passed through the Strat-M in 48 h was 149 ± 2 μg/cm2 for PC, 206 ± 4 μg/cm2 for CTAB/PC, 236 ± 2 μg/cm2 for PR-16/PC, 246 ± 2 μg/cm2 for IAC-16(Bu)/PC, and 257 ± 2 μg/cm2 for IA-16(OH)/PC liposomes.
For KP, transdermal drug permeation is enhanced as follows: PC < CTAB/PC < PR-16/PC < IAC-16(Bu)/PC < IA-16(OH)/PC. The total amount of KP that passed through the membrane in 48 h was 101 ± 2 μg/cm2 for PC, 112 ± 3 μg/cm2 for CTAB/PC, 155 ± 2 μg/cm2 for PR-16/PC, 169 ± 2 μg/cm2 for IAC-16(Bu)/PC, and 186 ± 3 μg/cm2 for IA-16(OH)/PC liposomes.
These results indicate that cationic surfactants in the liposome composition directly influence the permeation of drugs through the Strat-M. Probably, in this case, the adsorption/fusion mechanism of particle penetration is realized.28,64 In particular, the positive charge on the head groups of surfactants allows vesicles to adsorb on the skin surface, while the amphiphilic nature of surfactants allows them to act as edge activators. Hence, the vesicles are adsorbed on the skin surface and fuse with the lipid membrane in the corneum layer, and then, the drug molecule diffuses through the skin.64 The difference in the penetration of liposomes modified with different head groups is more likely directly connected with the different abilities of surfactants to loosen lipid bilayers. As mentioned earlier, edge activators are surfactants with a large radius of curvature causing destabilization of the lipid membrane and increasing its elasticity. Therefore, the higher the ability of surfactants to loosen the lipid bilayer, the more deformable the liposome can be. In earlier studies, the membranotropic ability of the studied amphiphiles (or similar in structures) was tested by changing the temperature phase transition of the model lipid (DPPC) in the presence of surfactants. It was shown that for the ammonium surfactant, incorporation into the lipid bilayer was the worst.65 The pyrrolidinium surfactant incorporated better than the ammonium analogue but worse than the imidazolium surfactants.30,35,38,66 The imidazolium series amphiphiles with various structural fragments (hydroxyethyl and carbamate) were incorporated at approximately the same level. This is probably the main reason why there is a considerable variation in the penetration of hybrid liposomes based on the surfactant with different head groups.
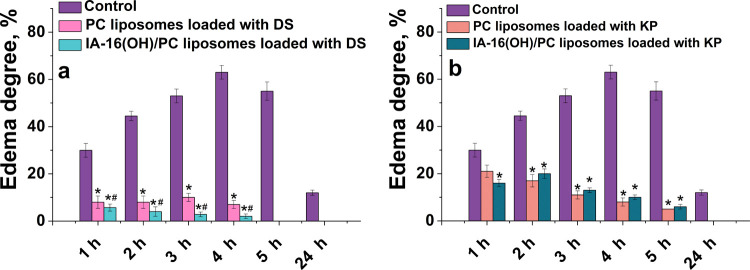
Furthermore, we proceed from in vitro to in vivo studies using leader liposomal formulations in terms of stability, decelerated release (optionally), and improved permeability. Consequently, DS- and KP-loaded IA-16(OH)/PC liposomes were selected, while unmodified PC formulations were used as a reference system. The anti-inflammatory ability was studied using a rat paw edema model induced using carrageenan. There were five groups of six animals in each one: control group without any treatment (1); two groups under treatment with DS (5 mg/mL) or KP (0.7 mg/mL) encapsulated in liposomes based on PC (2,3); and two groups under treatment with DS (5 mg/mL) or KP (0.7 mg/mL) loaded into IA-16(OH)/PC liposomes (4,5). Carrageenan was injected subcutaneously into the plantar rat paws, resulting in edema and an increase in the paw size. Furthermore, the rat paws were placed in a vessel with water, and the volume of the displaced liquid was measured. The paw volume measurements are shown in Tables S5 and S6. According to the data obtained, the degree of edema was also calculated as a percentage relative to the initial data presented in Figure 8.
Figure 8.
(a) Anti-edema effect of DS (a) and KP (b) formulations using the carrageenan-induced rat paw swelling model. Results presented as a mean ± SE of five animals in each group. *p < 0.05 compared to the control group of carrageenan-induced edema. #p < 0.05 compared to the PC liposomes loaded with the DS group of carrageenan-induced edema.
In groups treated with DS, an increase in rat paw edema over time was observed after the carrageenan injection. In the control group (without the drug treatment), 4 h after the carrageenan administration, the maximum increase in paw volume (by 63%) was observed. The paw volumes were significantly lower (p < 0.05) in the treatment with liposomal DS, compared with the control group (Table S5). In the case of treatment with DS (5 mg/mL)-loaded unmodified liposomes, the highest increase in the volume of the paw was observed after 3 h (11%). The edema was completely gone after 5 h. The IA-16(OH)/PC liposomes with DS (5 mg/mL) were the most effective. In this group of animals, the maximum increase in edema (5.7%) was observed an hour after the carrageenan injection, and the edema was completely gone after 5 h. Hybrid liposomes IA-16(OH)/PC with DS exhibited the largest anti-inflammatory activity (Figure 8a).
In groups treated with KP, in the group without the drug treatment, the maximum increase in the volume of the paw was detected 4 h after the carrageenan injection (by 63%). Treatment with liposomal KP made it possible to significantly reduce carrageenan-induced edema. The maximum increase in edema was observed after an hour (21%) and 2 h (20%) in the group treated with liposomal KP based on PC and IA-16(OH)/PC, respectively. Remarkably, the evident drug specificity occurred again in the in vivo assays. Unlike with liposomal DS, demonstrating a higher effect in the case of hybrid liposomes, the treatment with KP-loaded unmodified and hybrid liposomes shows practically similar inflammation inhibition. Importantly, in a recent in vivo study,30 a significantly higher anti-inflammatory effect of KP-loaded cationic liposomes modified with hydroxyethylated pyrrolidinium surfactants compared to PC liposomes has been reported. In light of these data, the key role of the structure of head groups rather than the occurrence of the positive charge should be emphasized.
4. Conclusions
Liposomal formulations for transdermal drug delivery based on phosphatidylcholine and cationic surfactants with various head groups were obtained for the first time. Diclofenac sodium, ketoprofen, and rhodamine B were used as substrates for encapsulation into liposomes. The hybrid liposomes were fabricated and optimized by varying the component molar ratio. Addition of cationic surfactants to PC liposomes improves the physicochemical properties and sustainability of the vesicles over time. In vitro monitoring of the drug release profile testified that prolonged release occurred in the case of hybrid formulations, with the pronounced effect of the medicines and surfactant structure observed. A comparative study of the ability of liposomes with different compositions for transdermal diffusion on Franz cells was performed. The liposomes modified using cationic surfactants with different head groups can significantly improve the transdermal drug penetration, which changes as follows: PC < CTAB/PC < PR-16/PC < IAC-16(Bu)/PC < IA-16(OH)/PC. Therefore, the most effective IA-16(OH)/PC liposomes were chosen for the in vivo study of the anti-inflammatory effect of KP and DS. A common model for such experiments is the carrageenan-induced rat paw edema. The treatment with KP-loaded unmodified and hybrid liposomes demonstrated practically similar inflammation inhibition. Conversely, DS-loaded hybrid liposomes, IA-16(OH)/PC, exhibited the highest anti-inflammatory activity. Maximum increases in edema of 11 and 5.7% were observed in the cases of unmodified and hybrid liposomes, respectively, and the edema was completely gone 5 h after the carrageenan injection.
Thus, for the first time, the structural factor was elucidated that controls the functional activity of liposomal formulations upon their modification with cationic surfactants to impart them affinity to the skin surface. Meanwhile, the most effective modifier IA-16(OH) has demonstrated superior properties in previous studies, e.g., this hydroxyethylated imidazolium surfactant showed improved membranotropic activity compared to CTAB and unsubstituted imidazolium analogues.35 Imidazolium surfactants are documented to possess a specific charge character of the head group, which is responsible for their effective complexation with DNA oligomers in the way differing from other surfactants, with the electrostatic mechanism neglected.38 In ref (12), IA-16(OH)-modified liposomes exhibited a prolonged release profile and low hemolytic activity and were successfully used as nanocarriers for cisplatin, exerting higher cytotoxicity toward M-HeLa cells compared to free drugs.
Acknowledgments
This work was financially supported by the Russian Science Foundation (project No 19-73-30012).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03039.
Electrophoretic mobility for empty liposomes; physicochemical characteristics of PC liposomes and liposomes modified using cationic surfactants loaded with RhB, DS, or KP; effect of formulations using the carrageenan-induced rat paw swelling model; extinction coefficient of diclofenac sodium and ketoprofen; and absorption spectra of RhB, DS, or KP (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ye R.; Yang J.; Li Y.; Zheng Y.; Yang J.; Li Y.; Liu B.; Jiang L. Fabrication of Tip-Hollow and Tip-Dissolvable Microneedle Arrays for Transdermal Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 2487–2494. 10.1021/acsbiomaterials.0c00120. [DOI] [PubMed] [Google Scholar]
- Huang D.; Sun M.; Bu Y.; Luo F.; Lin C.; Lin Z.; Weng Z.; Yang F.; Wu D. Microcapsule-Embedded Hydrogel Patches for Ultrasound Responsive and Enhanced Transdermal Delivery of Diclofenac Sodium. J. Mater. Chem. B 2019, 7, 2330–2337. 10.1039/C8TB02928H. [DOI] [PubMed] [Google Scholar]
- Momekova D. B.; Gugleva V. E.; Petrov P. D. Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery. ACS Omega 2021, 6, 33265–33273. 10.1021/acsomega.1c05083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K.; Thakkar H. P. Vinpocetine Loaded Ultradeformable Liposomes as Fast Dissolving Microneedle Patch: Tackling Treatment Challenges of Dementia. Eur. J. Pharm. Biopharm. 2020, 156, 176–190. 10.1016/j.ejpb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- Jijie R.; Barras A.; Boukherroub R.; Szunerits S. Nanomaterials for Transdermal Drug Delivery: Beyond the State of the Art of Liposomal Structures. J. Mater. Chem. B 2017, 5, 8653–8675. 10.1039/C7TB02529G. [DOI] [PubMed] [Google Scholar]
- Dragicevic-Curic N.; Fahr A. Liposomes in Topical Photodynamic Therapy. Expert Opin. Drug Delivery 2012, 9, 1015–1032. 10.1517/17425247.2012.697894. [DOI] [PubMed] [Google Scholar]
- Kotla N. G.; Chandrasekar B.; Rooney P.; Sivaraman G.; Larrañaga A.; Krishna K. V.; Pandit A.; Rochev Y. Biomimetic Lipid-Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272. 10.1021/acsbiomaterials.6b00681. [DOI] [PubMed] [Google Scholar]
- Alvarez-Román R.; Naik A.; Kalia Y. N.; Fessi H.; Guy R. H. Visualization of Skin Penetration Using Confocal Laser Scanning Microscopy. Eur. J. Pharm. Biopharm. 2004, 58, 301–316. 10.1016/j.ejpb.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Lasch J.; Laub R.; Wohlrab W. How Deep Do Intact Liposomes Penetrate into Human Skin?. J. Controlled Release 1992, 18, 55–58. 10.1016/0168-3659(92)90211-9. [DOI] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Ahtamyanova L. R.; Nizameev I. R.; Kadirov M. K.; Zakharova L. Ya. Novel Hybrid Liposomal Formulations Based on Imidazolium-Containing Amphiphiles for Drug Encapsulation. Colloids Surf., B 2019, 178, 352–357. 10.1016/j.colsurfb.2019.03.025. [DOI] [PubMed] [Google Scholar]
- Kuznetsova D. A.; Gaynanova G. A.; Vasileva L. A.; Sibgatullina G. V.; Samigullin D. V.; Sapunova A. S.; Voloshina A. D.; Galkina I. V.; Petrov K. A.; Zakharova L. Ya. Mitochondria-Targeted Cationic Liposomes Modified with Alkyltriphenylphosphonium Bromides Loaded with Hydrophilic Drugs: Preparation, Cytotoxicity and Colocalization Assay. J. Mater. Chem. B 2019, 7, 7351–7362. 10.1039/C9TB01853K. [DOI] [PubMed] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Gaynanova G. A.; Vasileva L. A.; Kuznetsov D. M.; Lukashenko S. S.; Voloshina A. D.; Sapunova A. S.; Nizameev I. R.; Sibgatullina G. V.; Samigullin D. V.; Kadirov M. K.; Petrov K. A.; Zakharova L. Ya. Novel Biocompatible Liposomal Formulations for Encapsulation of Hydrophilic Drugs – Chloramphenicol and Cisplatin. Colloids Surf., A 2021, 610, 125673 10.1016/j.colsurfa.2020.125673. [DOI] [Google Scholar]
- Mirgorodskaya A. B.; Kuznetsova D. A.; Kushnazarova R. A.; Gabdrakhmanov D. R.; Zhukova N. A.; Lukashenko S. S.; Sapunova A. S.; Voloshina A. D.; Sinyashin O. G.; Mamedov V. A.; Zakharova L. Y. Soft Nanocarriers for New Poorly Soluble Conjugate of Pteridine and Benzimidazole: Synthesis and Cytotoxic Activity against Tumor Cells. J. Mol. Liq. 2020, 317, 114007 10.1016/j.molliq.2020.114007. [DOI] [Google Scholar]
- AL Shuwaili A. H.; Rasool B. K. A.; Abdulrasool A. A. Optimization of Elastic Transfersomes Formulations for Transdermal Delivery of Pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. 10.1016/j.ejpb.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Bagchi D.; Dutta S.; Singh P.; Chaudhuri S.; Pal S. K. Essential Dynamics of an Effective Phototherapeutic Drug in a Nanoscopic Delivery Vehicle: Psoralen in Ethosomes for Biofilm Treatment. ACS Omega 2017, 2, 1850–1857. 10.1021/acsomega.7b00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaie S.; Bakhshayesh A. R. D.; Ha J. W.; Hamishehkar H.; Kim K. H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. 10.3390/nano10020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangjit S.; Obata Y.; Sano H.; Kikuchi S.; Onuki Y.; Opanasopit P.; Ngawhirunpat T.; Maitani Y.; Takayama K. Menthosomes, Novel Ultradeformable Vesicles for Transdermal Drug Delivery: Optimization and Characterization. Biol. Pharm. Bull. 2012, 35, 1720–1728. 10.1248/bpb.b12-00343. [DOI] [PubMed] [Google Scholar]
- Bishnoi S.; Rehman S.; Dutta S. B.; De S. K.; Chakraborty A.; Nayak D.; Gupta S. Optical-Property-Enhancing Novel Near-Infrared Active Niosome Nanoformulation for Deep-Tissue Bioimaging. ACS Omega 2021, 6, 22616–22624. 10.1021/acsomega.1c02632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H.; Kim A.; Oh Y.-K.; Kim C.-K. Effect of Edge Activators on the Formation and Transfection Efficiency of Ultradeformable Liposomes. Biomaterials 2005, 26, 205–210. 10.1016/j.biomaterials.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Elsayed M. M. A.; Abdallah O. Y.; Naggar V. F.; Khalafallah N. M. Deformable Liposomes and Ethosomes: Mechanism of Enhanced Skin Delivery. Int. J. Pharm. 2006, 322, 60–66. 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Sharma V. K.; Sarwa K. K.; Mazumder B. Fluidity Enhancement: A Critical Factor for Performance of Liposomal Transdermal Drug Delivery System. J. Liposome Res. 2014, 24, 83–89. 10.3109/08982104.2013.847956. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Cheng M.; Zhao J.; Zhang X.; Huang Z.; Zang Y.; Ding Y.; Zhang J.; Ding Z. Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines 2021, 9, 592. 10.3390/biomedicines9060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.; Dwivedi S.; Ajazuddin; Giri T. K.; Saraf S.; Saraf S.; Tripathi D. K. Approaches for Breaking the Barriers of Drug Permeation through Transdermal Drug Delivery. J. Controlled Release 2012, 164, 26–40. 10.1016/j.jconrel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Pegoraro C.; MacNeil S.; Battaglia G. Transdermal Drug Delivery: From Micro to Nano. Nanoscale 2012, 4, 1881. 10.1039/c2nr11606e. [DOI] [PubMed] [Google Scholar]
- Sinico C.; Manconi M.; Peppi M.; Lai F.; Valenti D.; Fadda A. M. Liposomes as Carriers for Dermal Delivery of Tretinoin: In Vitro Evaluation of Drug Permeation and Vesicle–Skin Interaction. J. Controlled Release 2005, 103, 123–136. 10.1016/j.jconrel.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Katahira N.; Murakami T.; Kugai S.; Yata N.; Takano M. Enhancement of Topical Delivery of a Lipophilic Drug from Charged Multilamellar Liposomes. J. Drug Targeting 1999, 6, 405–414. 10.3109/10611869908996847. [DOI] [PubMed] [Google Scholar]
- Manosroi A.; Kongkaneramit L.; Manosroi J. Stability and Transdermal Absorption of Topical Amphotericin B Liposome Formulations. Int. J. Pharm. 2004, 270, 279–286. 10.1016/j.ijpharm.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Duangjit S.; Opanasopit P.; Rojanarata T.; Ngawhirunpat T. Evaluation of Meloxicam-Loaded Cationic Transfersomes as Transdermal Drug Delivery Carriers. AAPS PharmSciTech 2013, 14, 133–140. 10.1208/s12249-012-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangjit S.; Opanasopit P.; Rojanarata T.; Ngawhirunpat T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug Delivery 2011, 2011, 1–9. 10.1155/2011/418316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet A.; Compère P.; Lecomte F.; Hubert P.; Ducat E.; Evrard B.; Piel G. Liposome Surface Charge Influence on Skin Penetration Behaviour. Int. J. Pharm. 2011, 411, 223–231. 10.1016/j.ijpharm.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Carrer D. C.; Vermehren C.; Bagatolli L. A. Pig Skin Structure and Transdermal Delivery of Liposomes: A Two Photon Microscopy Study. J. Controlled Release 2008, 132, 12–20. 10.1016/j.jconrel.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Gaynanova G.; Vasileva L.; Kashapov R.; Kuznetsova D.; Kushnazarova R.; Tyryshkina A.; Vasilieva E.; Petrov K.; Zakharova L.; Sinyashin O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. 10.3390/molecules26226786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova D. A.; Vasileva L. A.; Gaynanova G. A.; Vasilieva E. A.; Lenina O. A.; Nizameev I. R.; Kadirov M. K.; Petrov K. A.; Zakharova L. Ya.; Sinyashin O. G. Cationic Liposomes Mediated Transdermal Delivery of Meloxicam and Ketoprofen: Optimization of the Composition, in Vitro and in Vivo Assessment of Efficiency. Int. J. Pharm. 2021, 605, 120803 10.1016/j.ijpharm.2021.120803. [DOI] [PubMed] [Google Scholar]
- Samarkina D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Khamatgalimov A. R.; Kovalenko V. I.; Zakharova L. Ya. Cationic Amphiphiles Bearing Imidazole Fragment: From Aggregation Properties to Potential in Biotechnologies. Colloids Surf., A 2017, 529, 990–997. 10.1016/j.colsurfa.2017.07.018. [DOI] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Zakharova L. Ya. Adsorption and Membranotropic Properties of Colloid Systems Based on Cationic Amphiphiles: The Effect of the Head Group Structure. Russ. J. Phys. Chem. A 2019, 93, 1584–1588. 10.1134/S0036024419080168. [DOI] [Google Scholar]
- Gabdrakhmanov D. R.; Vasilieva E. A.; Voronin M. A.; Kuznetsova D. A.; Valeeva F. G.; Mirgorodskaya A. B.; Lukashenko S. S.; Zakharov V. M.; Mukhitov A. R.; Faizullin D. A.; Salnikov V. V.; Syakaev V. V.; Latypov S. K.; Zuev Y. F.; Zakharova L. Ya. Soft Nanocontainers Based on Hydroxyethylated Geminis: Role of Spacer in Self-Assembling, Solubilization, and Complexation with Oligonucleotide. J. Phys. Chem. C 2020, 124, 2178–2192. 10.1021/acs.jpcc.9b10079. [DOI] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Voloshina A. D.; Sapunova A. S.; Kulik N. V.; Nizameev I. R.; Kadirov M. K.; Kashapov R. R.; Zakharova L. Ya. Supramolecular Systems Based on Cationic Imidazole-Containing Amphiphiles Bearing Hydroxyethyl Fragment: Aggregation Properties and Functional Activity. J. Mol. Liq. 2019, 289, 111058 10.1016/j.molliq.2019.111058. [DOI] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Voloshina A. D.; Sapunova A. S.; Kashapov R. R.; Zakharova L. Ya. Self-Assembled Systems Based on Novel Hydroxyethylated Imidazolium-Containing Amphiphiles: Interaction with DNA Decamer, Protein and Lipid. Chem. Phys. Lipids 2019, 223, 104791 10.1016/j.chemphyslip.2019.104791. [DOI] [PubMed] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Kuznetsov D. M.; Lukashenko S. S.; Sapunova A. S.; Voloshina A. D.; Nizameev I. R.; Kadirov M. K.; Zakharova L. Ya. Biocompatible Supramolecular Systems Based on Novel Cationic Imidazolium- and Urethane-Containing Amphiphiles: Self-Assembly and Antimicrobial Properties. J. Mol. Liq. 2020, 319, 114094 10.1016/j.molliq.2020.114094. [DOI] [Google Scholar]
- Vasilieva E. A.; Lukashenko S. S.; Voloshina A. D.; Strobykina A. S.; Vasileva L. A.; Zakharova L. Ya. The Synthesis and Properties of Homologous Series of Surfactants Containing the Pyrrolidinium Head Group with Hydroxyethyl Moiety. Russ. Chem. Bull. 2018, 67, 1280–1286. 10.1007/s11172-018-2213-5. [DOI] [Google Scholar]
- Kuznetsova D. A.; Vasileva L. A.; Gaynanova G. A.; Pavlov R. V.; Sapunova A. S.; Voloshina A. D.; Sibgatullina G. V.; Samigullin D. V.; Petrov K. A.; Zakharova L. Ya.; Sinyashin O. G. Comparative Study of Cationic Liposomes Modified with Triphenylphosphonium and Imidazolium Surfactants for Mitochondrial Delivery. J. Mol. Liq. 2021, 330, 115703 10.1016/j.molliq.2021.115703. [DOI] [Google Scholar]
- Zakharova L. Ya.; Voloshina A. D.; Ibatullina M. R.; Zhiltsova E. P.; Lukashenko S. S.; Kuznetsova D. A.; Kutyreva M. P.; Sapunova A. S.; Kufelkina A. A.; Kulik N. V.; Kataeva O.; Ivshin K. A.; Gubaidullin A. T.; Salnikov V. V.; Nizameev I. R.; Kadirov M. K.; Sinyashin O. G. Self-Assembling Metallocomplexes of the Amphiphilic 1,4-Diazabicyclo[2.2.2]Octane Derivative as a Platform for the Development of Nonplatinum Anticancer Drugs. ACS Omega 2022, 7, 3073–3082. 10.1021/acsomega.1c06465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafiatullin B. Kh.; Radaev D. D.; Osipova M. V.; Sultanova E. D.; Burilov V. A.; Solovieva S. E.; Antipin I. S. Amphiphilic N-Oligoethyleneglycol-Imidazolium Derivatives of p-Tert-Butylthiacalix[4]Arene: Synthesis, Aggregation and Interaction with DNA. Macroheterocycles 2021, 14, 171–179. 10.6060/mhc210439s. [DOI] [Google Scholar]
- Samarkina D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Nizameev I. R.; Kadirov M. K.; Zakharova L. Ya. Homologous Series of Amphiphiles Bearing Imidazolium Head Group: Complexation with Bovine Serum Albumin. J. Mol. Liq. 2019, 275, 232–240. 10.1016/j.molliq.2018.11.082. [DOI] [Google Scholar]
- Kuznetsova D. A.; Kuznetsov D. M.; Amerhanova S. K.; Buzmakova E. V.; Lyubina A. P.; Syakaev V. V.; Nizameev I. R.; Kadirov M. K.; Voloshina A. D.; Zakharova L. Ya. Cationic Imidazolium Amphiphiles Bearing a Methoxyphenyl Fragment: Synthesis, Self-Assembly Behavior, and Antimicrobial Activity. Langmuir 2022, 38, 4921–4934. 10.1021/acs.langmuir.2c00299. [DOI] [PubMed] [Google Scholar]
- Kuznetsov D. M.; Kuznetsova D. A.; Gabdrakhmanov D. R.; Lukashenko S. S.; Nikitin Y. N.; Zakharova L. Y. Triallyl Ammonium Amphiphiles: Self-Assembly and Complexation with Bovine Serum Albumin. Surf. Innovations 2022, 10, 298–311. 10.1680/jsuin.21.00044. [DOI] [Google Scholar]
- Sultanova E.; Gazalieva A.; Makarov E.; Belov R.; Evtugyn V.; Burilov V.; Solovieva S.; Antipin I. Novel Aminocalixarene-Modified Polydiacetylene Vesicles: Synthesis and Naked-Eye Detection of ATP. Colloids Surf., A 2021, 630, 127642 10.1016/j.colsurfa.2021.127642. [DOI] [Google Scholar]
- Venter J. P.; Müller D. G.; du Plessis J.; Goosen C. A Comparative Study of an in Situ Adapted Diffusion Cell and an in Vitro Franz Diffusion Cell Method for Transdermal Absorption of Doxylamine. Eur. J. Pharm. Sci. 2001, 13, 169–177. 10.1016/S0928-0987(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Winter C. A.; Risley E. A.; Nuss G. W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Exp. Biol. Med. 1962, 111, 544–547. 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Su R.; Fan W.; Yu Q.; Dong X.; Qi J.; Zhu Q.; Zhao W.; Wu W.; Chen Z.; Li Y.; Lu Y. Size-Dependent Penetration of Nanoemulsions into Epidermis and Hair Follicles: Implications for Transdermal Delivery and Immunization. Oncotarget 2017, 8, 38214–38226. 10.18632/oncotarget.17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. Particle Size of Liposomes Influences Dermal Delivery of Substances into Skin. Int. J. Pharm. 2003, 258, 141–151. 10.1016/S0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- Todd P. A.; Sorkin E. M. Diclofenac Sodium: A Reappraisal of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1988, 35, 244–285. 10.2165/00003495-198835030-00004. [DOI] [PubMed] [Google Scholar]
- Scholer D. W.; Ku E. C.; Boettcher I.; Schweizer A. Pharmacology of Diclofenac Sodium. Am. J. Med. 1986, 80, 34–38. 10.1016/0002-9343(86)90077-X. [DOI] [PubMed] [Google Scholar]
- Menassé R.; Hedwall P. R.; Kraetz J.; Pericin C.; Riesterer L.; Sallmann A.; Ziel R.; Jaques R. Pharmacological Properties of Diclofenac Sodium and Its Metabolites. Scand. J. Rheumatol. 1978, 7, 5–16. 10.3109/03009747809097211. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh S.; Arami S. Enhanced Transdermal Delivery of Diclofenac Sodium via Conventional Liposomes, Ethosomes, and Transfersomes. BioMed Res. Int. 2013, 2013, 1–7. 10.1155/2013/616810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltner-Ukomadu E.; Sacha M.; Richter A.; Hussein K. Hydrogel Increases Diclofenac Skin Permeation and Absorption. Biopharm. Drug Dispos. 2019, 40, 217–224. 10.1002/bdd.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor T. G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacotherapy 1986, 6, 93–102. 10.1002/j.1875-9114.1986.tb03459.x. [DOI] [PubMed] [Google Scholar]
- Neupane R.; Boddu S. H. S.; Renukuntla J.; Babu R. J.; Tiwari A. K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. 10.3390/pharmaceutics12020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T.; Kadhum W. R.; Kanai S.; Todo H.; Oshizaka T.; Sugibayashi K. Prediction of Skin Permeation by Chemical Compounds Using the Artificial Membrane, Strat-M. Eur. J. Pharm. Sci. 2015, 67, 113–118. 10.1016/j.ejps.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Kaur L.; Singh K.; Paul S.; Singh S.; Singh S.; Jain S. K. A Mechanistic Study to Determine the Structural Similarities Between Artificial Membrane Strat-M and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. 10.1208/s12249-018-0959-6. [DOI] [PubMed] [Google Scholar]
- Simon A.; Amaro M. I.; Healy A. M.; Cabral L. M.; de Sousa V. P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with in Vivo-in Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. 10.1016/j.ijpharm.2016.08.052. [DOI] [PubMed] [Google Scholar]
- Cevc G. Transfersomes, Liposomes and Other Lipid Suspensions on the Skin: Permeation Enhancement, Vesicle Penetration, and Transdermal Drug Delivery. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 257–388. 10.1615/critrevtherdrugcarriersyst.v13.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Guo J.; Ping Q.; Sun G.; Jiao C. Lecithin Vesicular Carriers for Transdermal Delivery of Cyclosporin A. Int. J. Pharm. 2000, 194, 201–207. 10.1016/S0378-5173(99)00361-0. [DOI] [PubMed] [Google Scholar]
- Sala M.; Diab R.; Elaissari A.; Fessi H. Lipid Nanocarriers as Skin Drug Delivery Systems: Properties, Mechanisms of Skin Interactions and Medical Applications. Int. J. Pharm. 2018, 535, 1–17. 10.1016/j.ijpharm.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Gabdrakhmanov D. R.; Valeeva F. G.; Samarkina D. A.; Lukashenko S. S.; Mirgorodskaya A. B.; Zakharova L. Ya. The First Representative of Cationic Amphiphiles Bearing Three Unsaturated Moieties: Self-Assembly and Interaction with Polypeptide. Colloids Surf. Physicochem. Eng. Asp. 2018, 558, 463–469. 10.1016/j.colsurfa.2018.09.008. [DOI] [Google Scholar]
- Kuznetsova D. A.; Gabdrakhmanov D. R.; Ahtamyanova L. R.; Lukashenko S. S.; Kusova A. M.; Zuev Y. F.; Voloshina A. D.; Sapunova A. S.; Kulik N. V.; Kuznetsov D. M.; Nizameev I. R.; Kadirov M. K.; Zakharova L. Y. Novel Self-Assembling Systems Based on Imidazolium Amphiphiles with Cleavable Urethane Fragment for Construction of Soft Nanocontainers for Biomedicine Application. J. Mol. Liq. 2020, 298, 111961 10.1016/j.molliq.2019.111961. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.