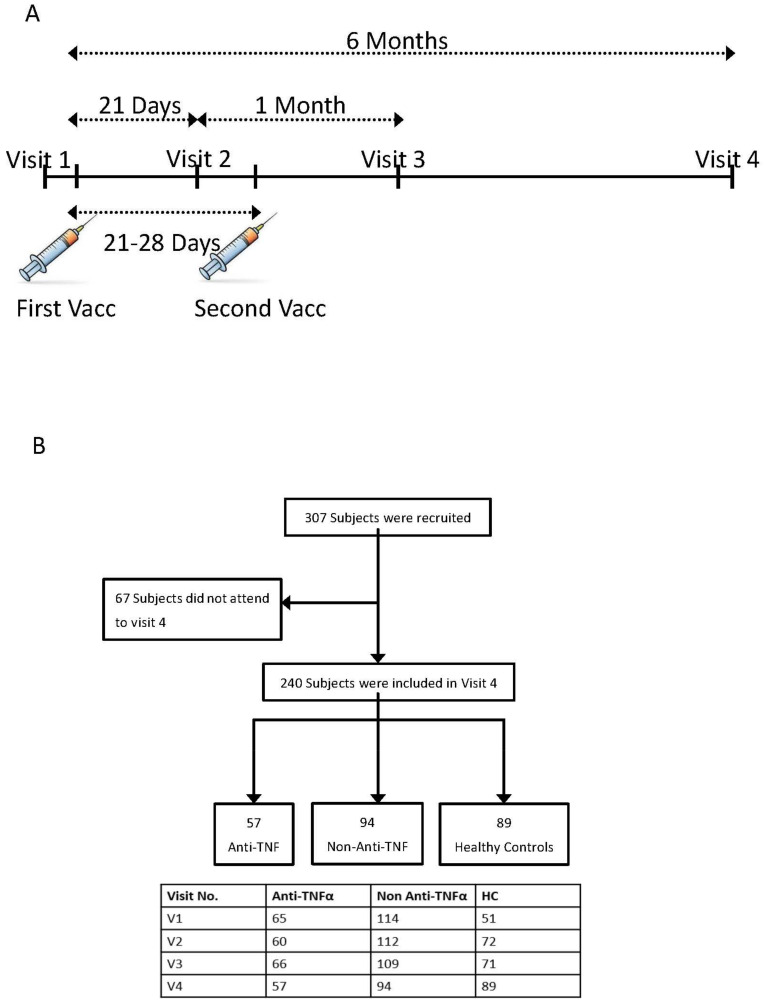
Figure 1.
(A) Study protocol. Patients were enrolled at visit 1, before the first vaccine dose. Visit 2 was 14–21 days after the first but before the second vaccine dose. Visit 3 and 4 were one and 6 months after the first vaccine dose, respectively. In each visit, laboratory tests were performed and questionnaires regarding disease severity and adverse events (AEs) were filled. (B) Patient disposition. The diagram represents all enrolled participants who were recruited before vaccination. In total, 25 subjects were recruited at the second visit (after first vaccine dose but before the second one), mainly due to logistic reasons. Most of them (19) were healthy controls (HCs). Number of subjects at each visit is detailed in the table below the diagram. Abbreviations: HC—healthy control; Vacc—vaccine dose.