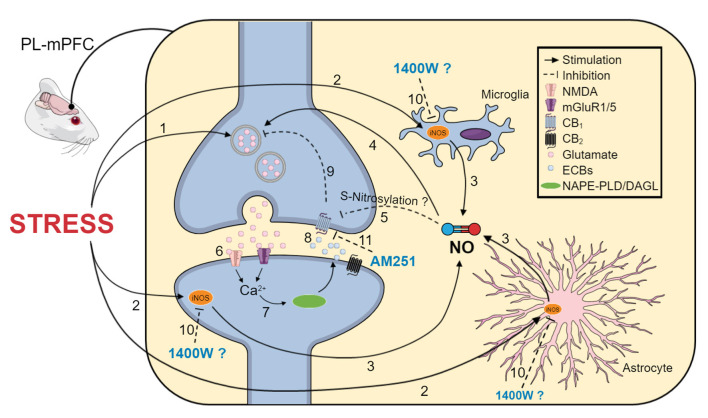
Figure 5.
Schematic representation of the proposed mechanisms involved in the anti-stress effect of 1400 W in the PL-mPFC. (1) Acute stress induces glutamate release (2) and activates iNOS, which could be expressed in microglia, astrocytes, and neurons. (3) Increased iNOS activity increases NO levels, (4) which can potentiate glutamatergic transmission. (5) NO may promote protein S-nitrosylation, including the CB1 receptor. Therefore, it could inhibit its function, impairing neurotransmission control. (6) Glutamate also activates mGlu5 and NMDA receptors, increasing intracellular calcium levels, (7) resulting in activation of the ECB synthesizing enzymes, NAPE-PLD and DAGL-a, increasing ECB levels. (8) Anandamide and 2-AG could act on CB1/2 receptors (9), regulating neuronal excitability. In the context of stress, NO could overcome ECBs' stress-buffer actions, favoring the release of glutamate, excessive excitability, and anxiety-like behaviors. In this way, (10) iNOS inhibition by 1400W could attenuate this positive feedback in neurons and reduce inflammatory consequences of iNOS activation in glial cells. ECBs acting at CB1 receptors contributes to the anxiolytic effect of 1400 W because (11) blocking CB1 receptors with AM251 prevented the anti-stress effect of 1400 W. We propose that the anti-stress effect of pharmacological iNOS inhibition in the PL-mPFC is dependent on the local ECB signaling, mediated at least by the CB1 receptors. The figure was simplified. Therefore, not all cells and components of synapses and glial cells are depicted.