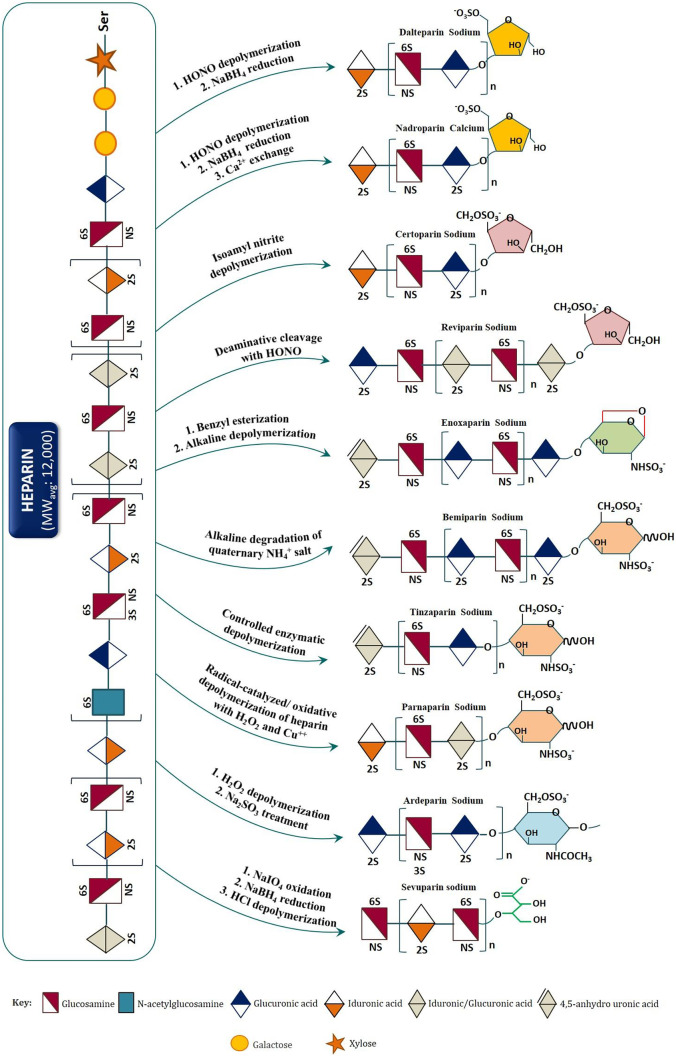
Fig. 2.
Scheme of depolymerization (enzymatic or chemical) used to prepare commercially available LMWHs from standard heparin. Signature structures (reducing end groups) are at the extremes right end. Identical groups are shown with same colors. (Sources: Wang Z. and Chi L. Recent advances in mass spectrometry analysis of low molecular weight heparins (2018). J. Chin. Chem. Lett., 29(1): 11–18; Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, Zhu W, Zhang C, Lu Y, Xing XH (2017). Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr Polym., 160:71–81. 10.1016/j.carbpol.2016.12.037; Fu L. et al. (2015). Bioengineered heparins and heparan sulfates, Adv. Drug Deliv. Rev., http://dx.doi.org/10.1016/j.addr.2015.11.002; Sánchez-Ferrer, C. F. (2010). Bemiparin. Drugs, 70, 19–23. 10.2165/1158581-s0-000000000-00000; Patents: US9475888; US10023659; WO2009007224A1; US9012229B2 (Assignee: Hangzhou Jiuyuan Gene Engineering Co., Ltd. and Shanghai Institute of Organic Chemistry, CAS); Web: Japanese Accepted Names (JAN) Name and Structure Database (National Institute of Health Sciences, https://jpdb.nihs.go.jp/jan/DetailList_en.aspx?submit=all_alp+Search&keyword=Reviparin+Sodium, accessed: April 20, 2021) Japanese Accepted Names for Pharmaceuticals (JAN Database) jpdb.nihs.go.jp)