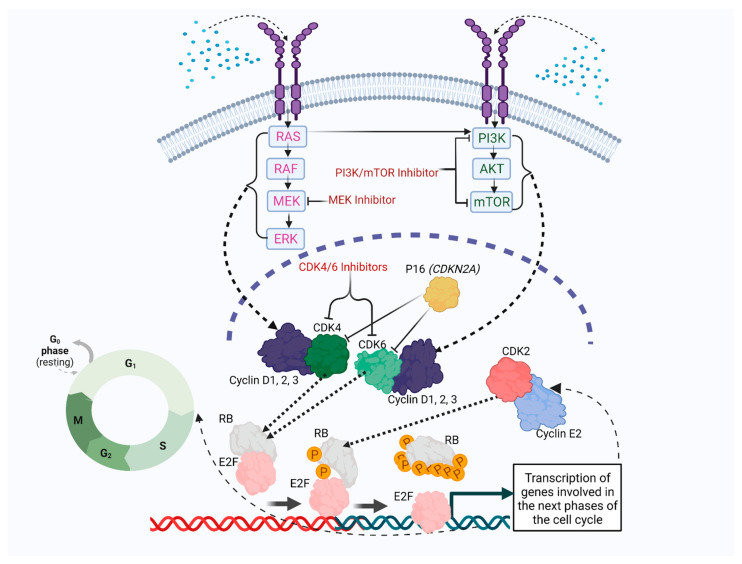
Figure 1.
Targeting dysregulation of Cyclin D–CDK4/6 axis by CDK4/6 inhibition. Cyclin D-CDK4/6-mediated RB phosphorylation occurs upon activation of Cyclin D-CDK4/6 complex in response to extracellular signals such as stimulatory mitogens, leading to ensuing cell cycle progression. Initially, upon Cyclin D-CDK4/6-mediated RB phosphorylation, RB affinity to E2F is reduced, promoting transcription of genes such as Cyclin E2. Subsequently, the Cyclin E2-CDK2 complex hyperphosphorylates RB, which induces full dissociation of the RB-E2F complex and transcription of genes encoding proteins playing critical roles in the next phases of the cell cycle. The p16INK4A (p16) tumor suppressor encoded by CDKN2A directly binds to CDK4/6 and inhibits the formation of the Cyclin D-CDK4/6 complex and prevents Cyclin D-CDK4/6-mediated RB phosphorylation. A dysregulated Cyclin D–CDK4/6 axis, which leads to uncontrolled cell proliferation, can be inhibited by CDK4/6 inhibitors. Chronic inhibition of CDK4/6 can trigger the upregulation of PI3K and MAPK pathways to compensate for inhibitory effects of CDK4/6 inhibitors on cell cycle progression by activation of D type cyclins. Dual inhibition of CDK4/6 and PI3K/mTOR or MEK may be an efficient combination treatment to prevent the compensatory effect of the PI3K or MAPK pathways on the development of CDK4/6 inhibitor resistance.