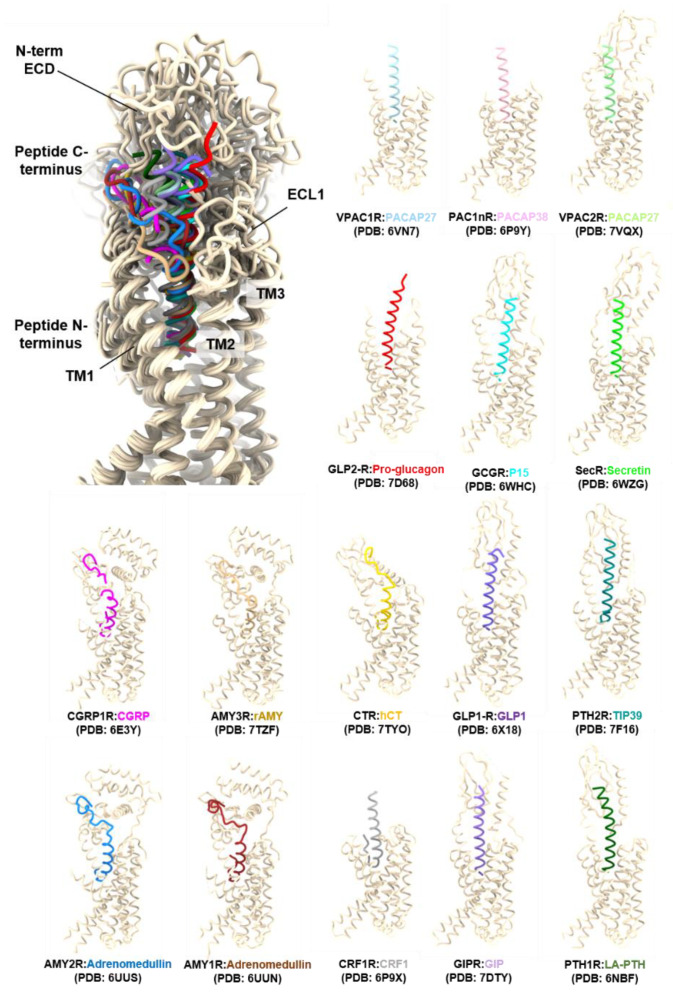
Figure 3.
Comparisons of the binding mode of representative peptides bound to Class B1 GPCRs reveal a shared peptide binding mode where the N-terminus of the peptide interacts with the transmembrane (TM) core and the C-terminus of the peptide interacts with the N-terminal extracellular domain (ECD) and extracellular loops (ECLs) of the receptor [135,136,137,300,301,302,303,304,305,306,307,308,309]. The highest variability of the structures is in the extracellular domains and peptide C-termini (as displayed in the structure overlay in the first panel. Structures were aligned by receptor chains and displayed as ribbons (licorice style) using Chimera X 1.3 [310,311]. G protein subunits are not displayed for clarity. The following models lack extracellular domains due to low resolution: PDB 6VN7, 6P9Y, 7D68, and 6P9X.