Table 5.
One-pot three-component synthesis and antibacterial activity of coumarin substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes 25.
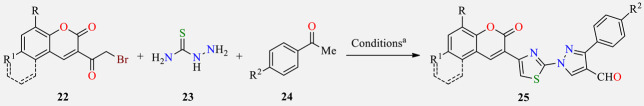
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R | R1 | R2 | Yield 25 (%) | MIC (μg/mL) | |||||
| EC | KP | PV | MRSA | BS | BC | |||||
| 25a | H | H | H | 84 | 135 | 150 | 133 | 150 | 132 | 145 |
| 25b | H | Cl | H | 80 | 134.6 | 129 | 130 | 150 | 129.5 | 138.6 |
| 25c | Cl | Cl | H | 75 | 119.6 | 118 | 131.5 | 115 | 123 | 117 |
| 25d | H | Br | H | 80 | 118.1 | 116.8 | 124 | 116 | 125 | 129 |
| 25e | Br | Br | H | 85 | 115.6 | 119 | 120 | 116.8 | 118 | 119 |
| 25f | H | Benzo | H | 78 | 132.8 | 150 | 150 | 135 | 122.8 | 138.9 |
| 25g | H | Cl | Cl | 77 | 126.3 | 117.4 | 130.7 | 134 | 112.2 | 110.8 |
| 25h | Cl | Cl | Cl | 73 | 110.9 | 105 | 113 | 112.5 | 110.7 | 112 |
| 25i | H | Br | Cl | 83 | 117.5 | 115 | 127.5 | 122.9 | 119.7 | 115 |
| 25j | MeO | H | Cl | 82 | 125.3 | 129 | 132.3 | 139 | 132.6 | 126 |
| 25k | H | H | Me | 77 | 132.5 | 150 | 150 | 150 | 150 | 135 |
| 25l | H | Br | Me | 82 | 134.6 | 124 | 121.8 | 138.1 | 125 | 126 |
| 25m | Cl | Cl | Me | 81 | 101.3 | 100.9 | 105 | 115.6 | 98.2 | 100.1 |
| 25n | Br | Br | Me | 85 | 86.5 | 79.1 | 100.7 | 105.9 | 92.4 | 72.8 |
| 25o | MeO | H | Me | 82 | 132.7 | 150 | 142 | 150 | 150 | 136 |
| Gentamycin b | -- | -- | -- | -- | 2 | 4 | 4 | 22 | 45 | 10 |
| Ampicillin b | -- | -- | -- | -- | 10 | -- c | -- c | 4 | 5 | 4 |
a Reaction conditions: 3-(2-bromoacetyl)coumarin derivative 22 (1 mmol), thiosemicarbazide 23 (1 mmol), substituted acetophenone 24 (1 mmol), DMF (5 mL), POCl3 (5 mmol), 0–60 °C, 5–6 h. b Positive control for the study. c Not determined. EC (Escherichia coli), KP (Klebsiella pneumoniae), PV (Proteus vulgaris), MRSA (Methicillin-resistant Staphylococcus aureus), BS (Bacillus subtilis), and BC (Bacillus cereus).
