Table 7.
One-pot three-component synthesis and antibacterial activity of 4-[(3-aryl-1-phenyl-1H-pyrazol-4-yl)methylidene]-2,4-dihydro-3H-pyrazol-3-ones 34.
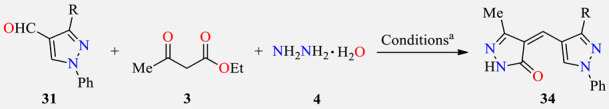
| ||||||
|---|---|---|---|---|---|---|
| Compound | R | Yield 34 (%) | Zone of Inhibition (mm) | |||
| SA | BS | PA | EC | |||
| 34a | C6H5 | 84 | 15.8 | 12.0 | 13.5 | 14.3 |
| 34b | 4-FC6H4 | 85 | 17.2 | 11.6 | 12.0 | 11.5 |
| 34c | 4-ClC6H4 | 88 | 14.0 | 8.9 | 10.9 | 10.8 |
| 34d | 4-BrC6H4 | 88 | 14.1 | 8.0 | 11.2 | 10.5 |
| 34e | 4-MeC6H4 | 90 | 9.0 | 8.4 | 9.6 | 10.2 |
| 34f | 4-MeOC6H4 | 92 | 10.0 | 7.9 | 8.8 | 9.2 |
| 34g | 3-MeOC6H4 | 85 | 12.2 | 9.4 | 12.0 | 8.0 |
| 34h | 4-OHC6H4 | 82 | 9.6 | 11.0 | 9.7 | 9.7 |
| 34i | 4-NO2C6H4 | 82 | 14.8 | 8.8 | 10.3 | 11.8 |
| 34j | 2,4-(Cl)2C6H3 | 86 | 15.6 | 10.8 | 12.1 | 12.0 |
| Norfloxacin b | -- | -- | 25.6 | 19.2 | 24.2 | 24.0 |
a Reaction conditions: 3-aryl-1-phenyl-1H-pyrazole-4-carbaldehyde 31 (1 mmol), ethyl acetoacetate 3 (1 mmol), hydrazine hydrate 4 (1 mmol), and sodium acetate (2 mmol), EtOH (5 mL), reflux, 1 h. b Positive control for the study. SA (Staphylococcus aureus), BS (Bacillus subtilis), PA (Pseudomonas aeruginosa), EC (Escherichia coli).
