Table 8.
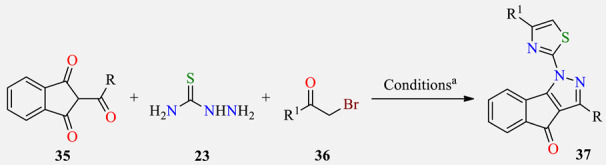
One-pot three-component synthesis of 3-alkyl-1-(4-(aryl/heteroaryl)thiazol-2- yl)indeno[1,2-c]pyrazol-4(1H)-ones 37 as antibacterial agents.
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | R1 | Yield 37 (%) | MIC (μmol/mL) | |||
| SA | BS | PA | EC | ||||
| 37a | Me | Biphenyl | 74 | 0.0297 | 0.0595 | 0.0297 | 0.0297 |
| 37b | Et | Biphenyl | 78 | 0.0288 | 0.0576 | 0.0288 | 0.0288 |
| 37c | iPr | Biphenyl | 72 | 0.0559 | 0.0559 | 0.0559 | 0.0559 |
| 37d | iBu | Biphenyl | 80 | 0.0270 | 0.0541 | 0.0270 | 0.0270 |
| 37e | Me | 2-Naphthyl | 72 | 0.0317 | 0.0635 | 0.0317 | 0.0317 |
| 37f | Et | 2-Naphthyl | 75 | 0.0613 | 0.0613 | 0.0306 | 0.0613 |
| 37g | iPr | 2-Naphthyl | 79 | 0.0593 | 0.0593 | 0.0593 | 0.0593 |
| 37h | iBu | 2-Naphthyl | 76 | 0.0287 | 0.0574 | 0.0287 | 0.0287 |
| 37i | Me | 2-Benzofuranyl | 63 | 0.0326 | 0.0652 | 0.0326 | 0.0326 |
| 37j | Et | 2-Benzofuranyl | 60 | 0.0629 | 0.0629 | 0.0629 | 0.0629 |
| 37k | iPr | 2-Benzofuranyl | 58 | 0.0607 | 0.0607 | 0.0607 | 0.0607 |
| 37l | iBu | 2-Benzofuranyl | 53 | 0.0293 | 0.0587 | 0.0293 | 0.0293 |
| Ciprofloxacin b | -- | -- | -- | 0.0094 | 0.0094 | 0.0094 | 0.0094 |
a Reaction conditions: 2-acyl-(1H)-indene-1,3-(2H)dione 35 (1 mmol), thiosemicarbazide 23 (1 mmol), MeOH (30 mL), reflux, 10–15 min, then α-bromoketone 36 (5 mmol), sodium acetate (5 mmol), MeOH/AcOH (2:1, v/v) (20 mL), reflux, 5–8 h. b Positive control for the study. SA (Staphylococcus aureus), BS (Bacillus subtilis), PA (Pseudomonas aeruginosa), EC (Escherichia coli).
