Table 14.
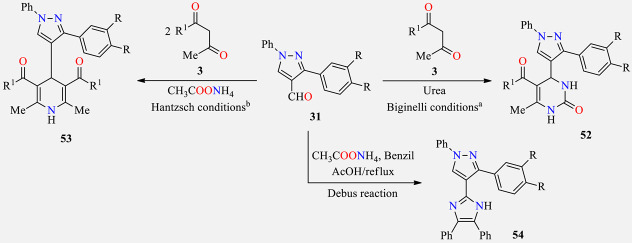
Multicomponent synthesis of pyrimidine-, dihydropyridine-, and imidazole-based pyrazoles 52, 53, and 54 and their in vitro antibacterial activity.
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | R1 | Yield of 52, 53, 54 (%) | Antibacterial Activity of the Synthesized Compounds 52, 53, 54(MIC in μg·mL−1) c | |||
| SA | EC | PA | KP | ||||
| 52a | Cl | OEt | 90 | 25 (13) | 25 (14) | NP | 50 (9) |
| 52b | Cl | Ome | 88 | 12.5 (15) | 12.5 (15) | 25 (10) | 25 (11) |
| 52c | Cl | Me | 77 | 6.25 (18) | 6.25 (18) | 6.25 (19) | 12.5 (12) |
| 52d | F | Oet | 89 | 12.5 (12) | 12.5 (14)0 | NP | 12.5 (11) |
| 52e | F | Ome | 80 | 25 (12) | 12.5 (13) | 50 (10) | 25 (11) |
| 52f | F | Me | 72 | 12.5 (16) | 6.25 (16) | 6.25 (17) | 6.25 (19) |
| 53a | Cl | Oet | 74 | 12.5 (14) | 12.5 (14) | 25 (11) | 25 (12) |
| 53b | Cl | Ome | 79 | 50 (9) | 25 (11) | NP | 100 (4) |
| 53c | Cl | Me | 64 | 50 (10) | 50 (11) | 50 (9) | NP |
| 53d | F | Oet | 72 | 25 (11) | 25 (10) | NP | 12.5 (12) |
| 53e | F | Ome | 65 | 12.5 (13) | 12.5 (15) | 25 (10) | 12.5 (15) |
| 53f | F | Me | 62 | 50 (7) | 50 (8) | 100 (8) | NP |
| 54a | Cl | -- | 86 | NP | 25 (12) | 12.5 (10) | NP |
| 54b | F | -- | 78 | 50 (10) | 25 (11) | 25 (9) | 100 (5) |
| Streptomycin d | -- | -- | 6.25 (20) | 6.25 (17) | 6.25 (19) | 6.25 (18) | |
a Biginelli reaction conditions: A mixture of compound 31 (2 mol), type keto-derivative 3 (2.2 mol), urea (3 mol), and HCl (0.5 mL) in ethanol was heated to reflux for 6 h. b Hantzsch reaction conditions: A mixture of compound 31 (1.0 mol), type keto-derivative 3 (2 mol), and ammonium acetate (1.1 mol) in ethanol (20 mL) was refluxed for 8 h. c Values in brackets correspond to zone of inhibition in mm. SA (Staphylococcus aureus), EC (Escherichia coli), PA (Pseudomonas aeruginosa), KP (Klebsiella pneumonia). d Positive control for the study.
