Table 28.
Four-component synthesis and anticancer activity of pyranopyrazole derivatives 96.
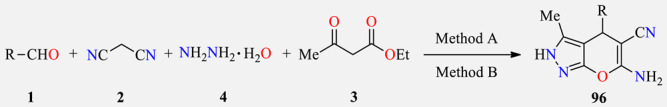
| ||||
|---|---|---|---|---|
| Compound | R | Yield 96 (%) | IC50 (μg/mL) | |
| Method A | Method B | Hep3B | ||
| 96a | 3-HOC6H4 | 88 | 82 | 32 |
| 96b | 4-BrC6H4 | 82 | 80 | 16 |
| 96c | 3-BrC6H4 | 80 | 77 | 10 |
| 96d | 3-NO2C6H4 | 86 | 81 | 32 |
| 96e | 3-Thiophenyl | 88 | 80 | 24 |
| 96f | 2-Pyrrolyl | 85 | 80 | 128 |
| 96g | 3-Indolyl | 81 | 79 | 64 |
| 96h | 4-ClC6H4 | 77 | 70 | 96 |
| 96i | 2-IC6H4 | 80 | 75 | 96 |
| 96j | C6H5 | 86 | 81 | 128 |
| 96k | n-Butyl | 82 | 83 | 20 |
| 96l | 4-MeC6H4 | 88 | 87 | 20 |
| 96m | 4-Pyridinyl | 94 | 85 | 48 |
| 96n | 2-FC6H4 | 90 | 87 | 32 |
Method A: Aldehyde 1 (2.2 mmol), malononitrile 2 (2.2 mmol), hydrazine hydrate 4 (2.0 mmol), ethyl acetoacetate 3 (2.0 mmol), Et3N (3.0 mmol), EtOH (4 mL), 1–2 h, r.t. Method B: Aldehyde 1 (2.2 mmol), malononitrile 2 (2.2 mmol), hydrazine hydrate 4 (2.0 mmol), ethyl acetoacetate 3 (2.0 mmol), Et3N (3.0 mmol), EtOH (4 mL), 3–5 min, 60 °C, MWI.
