Table 32.
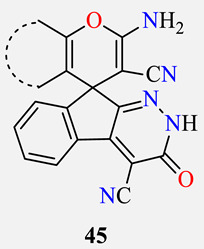
Evaluation of spiroindenopyridazine-4H-pyrans 45 against the cancer cell lines A549, PC-3, MCF-7, A375, LNCaP, and Normal cell HDF a.
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | Cyclic CH-Acid 44 in Table 10 |
IC50 (μM) | |||||
| A549 | PC-3 | MCF-7 | A375 | LNCaP | HDF | ||
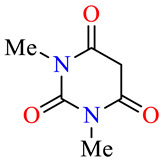
| 45a |
|
40 | >100 | >100 | 70.7 | 32.1 | >100 |
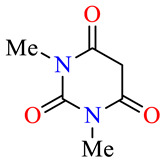
| 45b |
|
>100 | >100 | >100 | >100 | >100 | >100 |
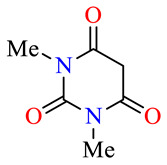
| 45c |
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45d |
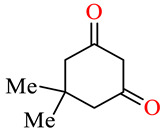
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45e |
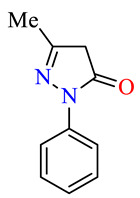
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45f |
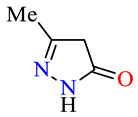
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45g |
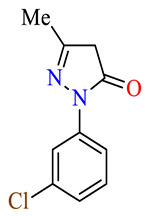
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45h |
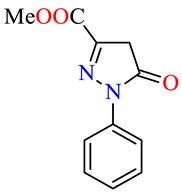
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45i |
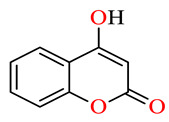
|
>100 | >100 | >100 | >100 | >100 | >100 |
| 45j |
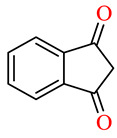
|
>100 | >100 | >100 | >100 | >100 | >100 |
| Etoposide b | -- | 60 | 40 | 30 | 25.3 | 90 | >100 |
a Reaction conditions: Cyanoacetohydrazide 42 (1 mmol), ninhydrin 43 (1 mmol), malononitrile 2 (1 mmol), cyclic CH-acid 44 (1 mmol), EtOH (10 mL), reflux, 6–12 h. b Standard drug for the study.
