Table 45.
Multicomponent Debus–Radziszewski synthesis of imidazolylpyrazoles 145 as α-glucosidase inhibitors.
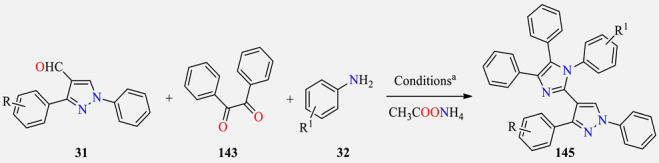
| |||||
|---|---|---|---|---|---|
| Compound | R | R1 | Yield of 145 (%) | α-Glucosidase Inhibition | |
| Percentage Inhibition (%) | IC50 (µM) | ||||
| 145a | 4-MeO | 4-MeO | 75 | 99.56 | 178.82 |
| 145b | 4-MeO | 4-Br | 70 | 91.12 | 162.93 |
| 145c | 4-MeO | 3,5-(Me)2 | 81 | 96.13 | 182.17 |
| 145d | 4-Cl | 4-MeO | 78 | 98.65 | 168.92 |
| 145e | 4-Cl | 4-Cl | 69 | 93.19 | 85.71 |
| 145f | 4-Cl | 4-Br | 73 | 96.21 | 25.19 |
| 145g | 4-Cl | 3,5-(Me)2 | 84 | 89.54 | 132.81 |
| 145h | 4-Br | 4-MeO | 82 | 89.76 | 104.75 |
| 145i | 4-Br | 4-Cl | 71 | 87.25 | 84.61 |
| 145j | 4-Br | 3,5-(Me)2 | 81 | 75.23 | 412.42 |
| 145k | 3-NO2 | 4-Cl | 77 | 96.76 | 42.23 |
| 145l | 3-NO2 | 4-MeO | 84 | 95.79 | 43.14 |
| 145m | 3-NO2 | 4-Br | 81 | 97.52 | 33.62 |
| 145n | 3-NO2 | 3,5-(Me)2 | 88 | 91.25 | 58.73 |
| Acarbose b | -- | -- | -- | 92.23 | 38.25 |
a Reaction conditions: Pyrazole-4-carbaldehydes 31, benzil 143, substituted anilines 32, and ammonium acetate, AcOH, MWI. b Reference compound.
