Table 51.
Ultrasound-assisted three-component synthesis of 1,2,4-triazol-1-yl-pyrazole-based spirooxindolopyrrolizidines 157 as antitubercular agents.
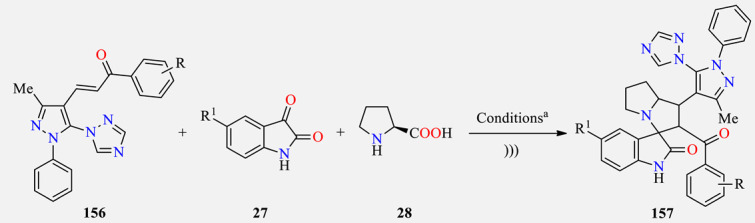
| |||||
|---|---|---|---|---|---|
| Compound | R | R1 | Yield 157 (%) | MIC (μg/mL) | Cytotoxicity % Inhibition at 25 μg/mL c |
| 157a | H | H | 92 | >25 | -- d |
| 157b | 4-Me | H | 83 | >25 | -- d |
| 157c | 4-MeO | H | 78 | 12.5 | -- d |
| 157d | 4-Cl | H | 86 | 6.25 | 16.85 |
| 157e | 4-Br | H | 84 | 1.56 | 27.15 |
| 157f | 3-NO2 | H | 92 | >25 | -- d |
| 157g | 4-NO2 | H | 90 | 6.25 | 29.61 |
| 157h | H | Cl | 89 | 0.78 | 19.76 |
| 157i | 4-Me | Cl | 80 | 25.0 | -- d |
| 157j | 4-MeO | Cl | 76 | 3.12 | 21.65 |
| 157k | 4-Cl | Cl | 82 | 1.56 | 17.91 |
| 157l | 4-Br | Cl | 81 | 1.56 | 26.43 |
| 157m | 3-NO2 | Cl | 90 | 6.25 | 18.96 |
| 157n | 4-NO2 | Cl | 88 | 1.56 | 24.94 |
| 157o | H | Br | 85 | 3.12 | 18.62 |
| 157p | 4-MeO | Br | 79 | 25.0 | -- d |
| 157q | 4-Cl | Br | 85 | 1.56 | 22.36 |
| 157r | 4-Br | Br | 80 | 1.56 | 21.97 |
| 157s | 3-NO2 | Br | 86 | 6.25 | 20.08 |
| 157t | 4-NO2 | Br | 85 | 6.25 | 26.40 |
| Ethambutol b | -- | -- | -- | 1.56 | -- d |
a Reaction conditions: Pyrazole-based chalcones 156 (1.0 mmol), substituted isatins 27 (1.0 mmol), and L-proline 28 (1.0 mmol) in [Bmim]BF4 (3.0 mL) at 60 °C for 6–16 min under ultrasound irradiation. b Reference compound. c The cytotoxicity was determined in the RAW 264.7 cell line. d Not determined.
