Table 54.
Three-component synthesis and in vitro antileishmanial activity of pyrazolodihydropyridines 164.
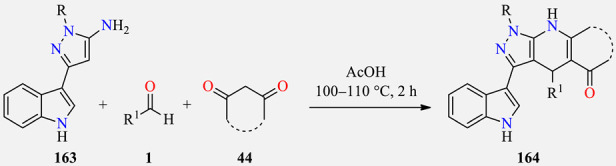
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | R1 | Reagent 44 | Promastigotes GI (%) |
Amastigotes GI (%) |
||
| 25 μM | 50 μM | 25 μM | 50 μM | ||||
| 164a | 4-ClC6H4 | 4-ClC6H4 |
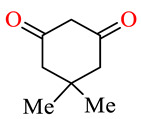
|
62.5 | 65.4 | NSI b | NSI b |
| 164b | 4-FC6H4 | 4-ClC6H4 |
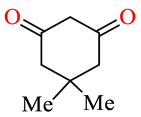
|
54.3 | 65.3 | NSI | NSI |
| 164c | 4-ClC6H4 | 3,4,5-(MeO)3C6H2 |
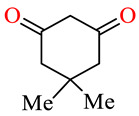
|
79.3 | 86.7 | 23.6 | 28.2 |
| 164d | 3,4-(Cl)2C6H3 | 2,5-(MeO)2C6H3 |
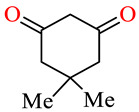
|
85.9 | 90.6 | 91.5 | 95.8 |
| 164e | 3,4-(Cl)2C6H3 | 3,4-(MeO)2C6H3 |
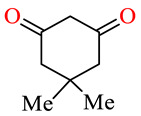
|
52.3 | 59.5 | NSI | NSI |
| 164f | 4-ClC6H4 | 2-Thiophenyl |
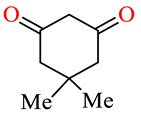
|
82.5 | 86.5 | 43.8 | 51.7 |
| 164g | 4-ClC6H4 | 2,5-(MeO)2C6H3 |
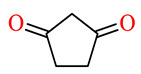
|
85.5 | 93.2 | 37.3 | 42.6 |
| 164h | 4-ClC6H4 | 3,4,5-(MeO)3C6H2 |
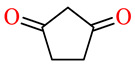
|
78.4 | 81.2 | 45.2 | 59.6 |
| 164i | 4-ClC6H4 | 4-OH-3,5-(MeO)2C6H2 |
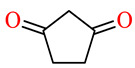
|
79.1 | 89.6 | 25.2 | 30.7 |
| 164j | 3,4-(Cl)2C6H3 | 3,4-(MeO)2C6H3 |
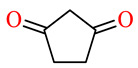
|
91.7 | 93.3 | 96.8 | 97.3 |
| 164k | 4-FC6H4 | 4-OH-3,5-(MeO)2C6H2 |
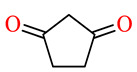
|
56.5 | 66.9 | NSI | NSI |
| 164l | 4-ClC6H4 | 4-ClC6H4 |
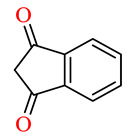
|
49.1 | 62.4 | NSI | NSI |
| 164m | 4-FC6H4 | 4-ClC6H4 |
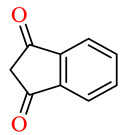
|
54.2 | 68.3 | NSI | NSI |
| 164n | 4-ClC6H4 | 2,3,4-(MeO)3C6H2 |
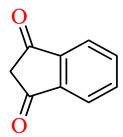
|
64.2 | 67.4 | NSI | NSI |
| 164o | 4-ClC6H4 | 2-Thiophenyl |
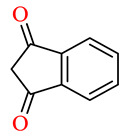
|
65.3 | 69.2 | NSI | NSI |
| Miltefosine a | -- | -- | -- | 100 | 100 | 99.8 | 100 |
a Reference compound. b NSI (no significant inhibition).
