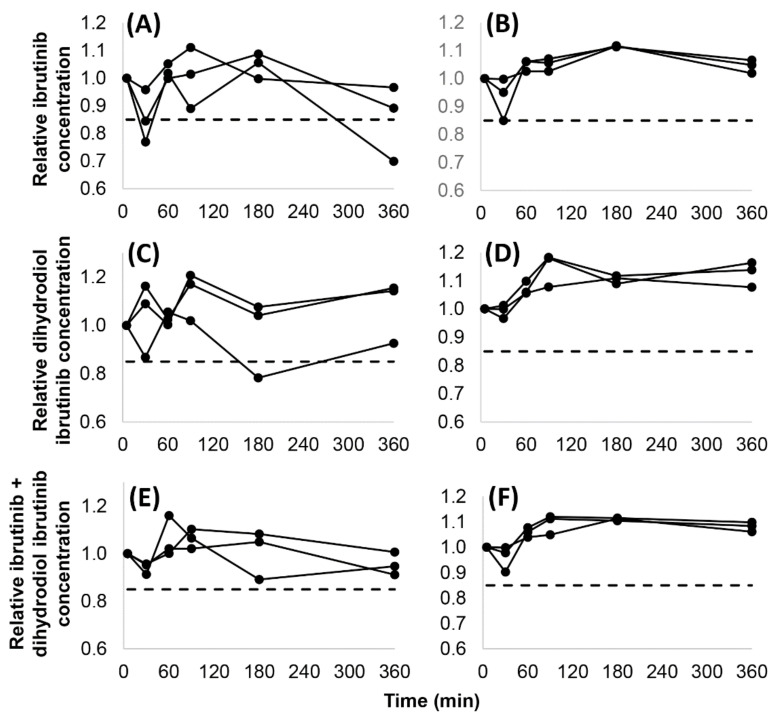
Figure 2.
Stability of ibrutinib (A,B), dihydrodiol ibrutinib (C,D) and the active moiety (sum of ibrutinib and dihydrodiol ibrutinib concentrations) (E,F) in whole blood (A,C,E) and in plasma (B,D,F) at 25 °C over 6 h in 3 independent samples. The dashed line (- - -) displays the limit for judging analyte stability as acceptable (0.85).