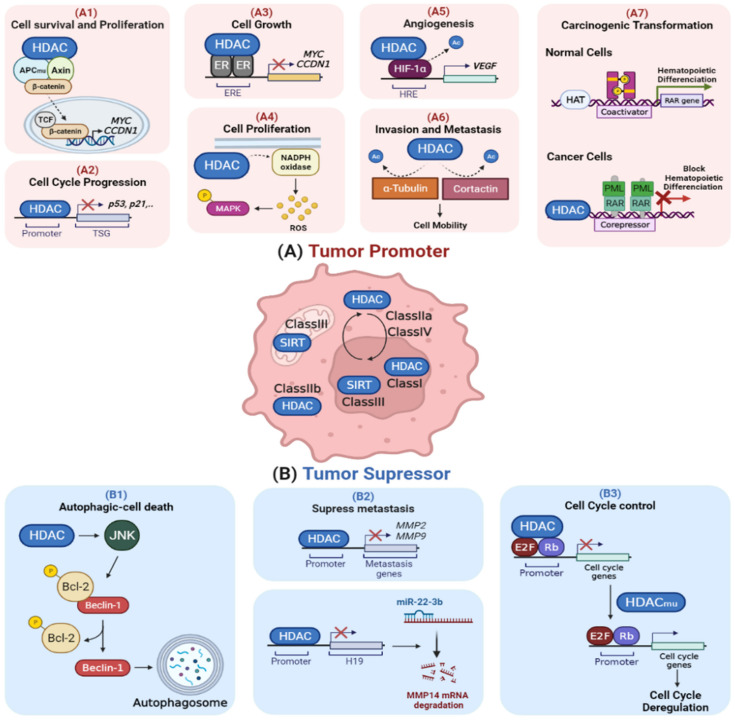
Figure 1.
HDACs as tumor promoters and suppressors. (A) HDACs are overexpressed in cancer, which promotes cellular proliferation and suppresses apoptosis and cell cycle arrest. (A1) Some HDACs (e.g., HDAC2) stabilize the beta-catenin complex, boosting cell survival and proliferation. (A2) HDACs are involved in the disruption of cell cycle checkpoints by obstructing the expression of tumor suppressor genes such as p53 and p21. (A3) Certain HDACs, such as HDAC1, reduce the transcription activity of estrogen receptor-α (ER-α), resulting in growth promotion. (A4) HDACs (e.g., HDAC6) increase the activation of MAPK through inducing the production of reactive oxygen species (ROS) via NADPH oxidase, thus promoting cell proliferation and survival. (A5) Under hypoxia, HDACs (e.g., HDAC1) stabilize HIF-1α through deacetylation, which, in turn, activates the transcription of genes involved in oxygen delivery, energy metabolism, angiogenesis, and apoptosis. (A6) The deacetylation of cell motility proteins (tubulin and cortactin) by HDACs (e.g., HDAC6) drives the progression of a primary tumor to invasion and metastasis. (A7) In hematological malignancies, PML-RAR oncofusion proteins act as altered transcription factors, which aberrantly recruit HDACs to the promoter site of retinoic acid (RA) genes, thus suppressing myeloid differentiation and causing malignant transformation. (B) Some HDACs have tumor suppressor activities and they are genetically downregulated in cancer. (B1) HDACs suppress tumor growth through activating JNK-mediated Beclin1 dissociation from Bcl-2 to induce caspase-independent autophagy death. (B2) HDACs (e.g., HDAC10) inhibit invasion and metastasis through reducing the histone acetylation level at the promoter sites of the matrix metalloproteinases (MMP2 and MMP9) genes, thereby suppressing their expression. Additionally, HDACs (e.g., HDAC2) suppress cancer metastasis through inhibiting expression of LncRNA H19, a miR-22-3P sponge that upregulates the expression of MMP14, by histone H3K27 deacetylation at its promoter site. (B3) Some HDACs (e.g., HDAC1) are involved in cell cycle regulation by forming a complex with E2F and RB that represses cell cycle progression genes. Mutations in HDAC1 reduce its recruitment and binding to E2F-regulated promoters, thereby reducing their interaction with retinoblastoma protein (Rb). This results in preventing the repression of cell cycle genes by retinoblastoma (Rb). Abbreviations: APC, adenomatous polyposis coli; TSG, tumor suppressor gene; ER, estrogen receptor; ERE, estrogen response element; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; HIF-1α, hypoxia-inducible factor-1; HRE, hypoxia-response element; VEGF, vascular endothelial growth factor; RAR, retinoic acid receptor; PML, promyelocytic leukemia; HAT, histone acetyltransferase; JNK, c-Jun N-terminal kinases; Bcl-2, B-cell lymphoma 2; MMP, matrix metalloproteinases; miR, microRNA; Rb, retinoblastoma.