Hydrogen evolution reaction (HER) on Pt single-crystal surfaces, in acid solutions, and the corresponding available data: Tafel slope (b), exchange current density (jo) at given temperatures (T), activation energy (Ea), number of electrons transferred (z) and identified mechanism and rate-determining step (rds).409 Modified from ref. 263. Copyright MDPI 2020.
| Single crystal | b (mV dec−1) | z | j o (mA cm−2) (T) | E a (kJ mol−1) | Mechanism and RDS |
|---|---|---|---|---|---|
| Pt(100) |
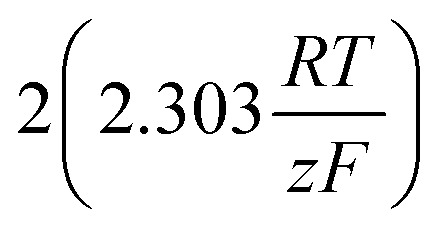
|
1 | 0.36 (274 K) | 12 | Heyrovsky–Volmer |
| 0.60 (303 K) | |||||
| 0.76 (333 K) | |||||
| Pt(110) |
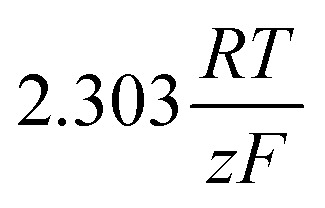
|
2 | 0.65 (274 K) | 9.5 | Tafel–Volmer |
| 0.98 (303 K) | |||||
| 1.35 (333 K) | |||||
| Pt(111) |
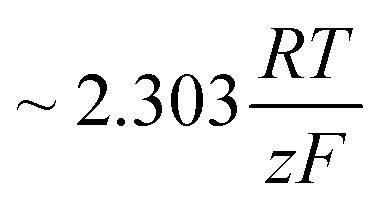
|
1 | 0.21 (274 K) | 18 | Tafel–Volmer, Heyrovsky–Volmer |
| 0.45 (303 K) | |||||
| 0.83 (333 K) |
