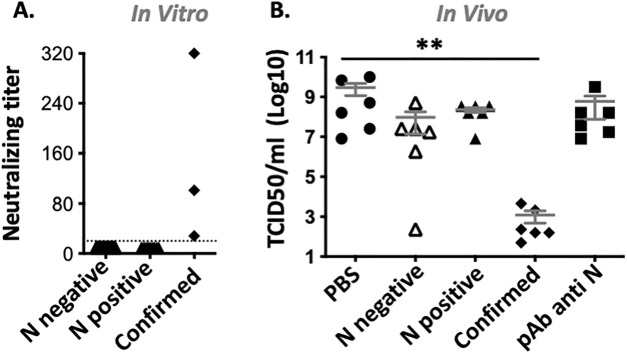
Figure 2.
In vitro and in vivo neutralization of human sera with antibodies against SARS-CoV-2 N protein. (A) Neutralising titer of sera from Gabonese donors with (N positive) or without (N negative) Ab against SARS-CoV-2 N protein (n = 10/group). Standard sera from COVID-19 confirmed cases with high, medium and low neutralization capacity were used as positive control. Sera with neutralizing titer below the lowest tested dilution (dotted line), are plotted at 10. (B) Day 5 lung viral titer (TCID50/ml) in SARS-CoV-2 challenged mice (n = 6/group) pretreated with either PBS; 1 mg purified IgG from Gabonese sera with (N positive) or without (N negative) Ab against SARS-CoV-2 N protein; or from COVID-19 confirmed cases (confirmed) or with 50 μg polyclonal antibodies against SARS-CoV-2 N (pAb anti N). Mean ± SEM is depicted.