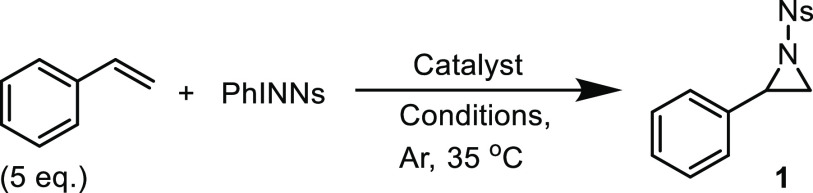
Table 1. Optimization of the Reaction Conditions for the Formation of 1, Catalyzed by PPh4[CoIII(TAMLred)] or [CoIII(TAMLsq)]e.
| entry | catalyst loading (mol %) | solvent | concentration PhINNs (mM) (time, h) | yield (%) |
|---|---|---|---|---|
| PPh4[CoIII(TAMLred)] | ||||
| 1 | 5.0 | toluene | 48 (16) | 40 |
| 2 | 5.0 | benzene | 48 (16) | 41 |
| 3 | 5.0 | MeCN | 48 (16) | 18 |
| 4 | 5.0 | CH2Cl2 | 48 (16) | 58 |
| 5a | 5.0 | CH2Cl2 | 48 (16) | 44 |
| 6 | 5.0 | CH2Cl2 | 24 (16) | 77 |
| 7 | 5.0 | CH2Cl2 | 24 (2) | 76 |
| 8 | 2.5 | CH2Cl2 | 24 (2) | 64 |
| 9b | 2.5 | CH2Cl2 | 24 (2) | 67 |
| 10c | – | CH2Cl2 | 24 (2) | 0 |
| [CoIII(TAMLsq)] | ||||
| 11 | 5.0 | toluene | 48 (16) | 7 |
| 12 | 5.0 | CH2Cl2 | 48 (16) | 57 |
| 13 | 5.0 | CH2Cl2 | 24 (2) | 74 |
| 14 | 2.5 | CH2Cl2 | 24 (2) | 35 |
| 15b | 2.5 | CH2Cl2 | 24 (2) | –d |
1.0 equiv styrene was used.
Aerobic conditions.
No catalyst added.
[CoIII(TAMLsq)] is not stable under aerobic conditions.
Yields based on 1H NMR integration using 1,3,5-trimethoxybenzene as an internal standard..