Abstract
Prosopis is a commercially important plant genus, which has been used since ancient times, particularly for medicinal purposes. Traditionally, Paste, gum, and smoke from leaves and pods are applied for anticancer, antidiabetic, anti-inflammatory, and antimicrobial purposes. Components of Prosopis such as flavonoids, tannins, alkaloids, quinones, or phenolic compounds demonstrate potentials in various biofunctions, such as analgesic, anthelmintic, antibiotic, antiemetic, microbial antioxidant, antimalarial, anti-protozoal, antipustule, and antiulcer activities; enhancement of H+, K+, ATPases; oral disinfection; and probiotic and nutritional effects; as well as in other biopharmaceutical applications, such as binding abilities for tablet production. The compound juliflorine provides a cure in Alzheimer disease by inhibiting acetylcholine esterase at cholinergic brain synapses. Some indirect medicinal applications of Prosopis spp. are indicated, including antimosquito larvicidal activity, chemical synthesis by associated fungal or bacterial symbionts, cyanobacterial degradation products, “mesquite” honey and pollens with high antioxidant activity, etc. This review will reveal the origins, distribution, folk
Keywords: medicinal application, natural product, Prosopis, secondary metabolite
1. Introduction
Small trees or shrubs that are representatives of Prosopis can be major plants component in drylands of Africa, America, and Asia. This plant is hardy, drought resistant, and fast growing [1]. The genus Prosopis accommodates 44 species, of which 40 are native to North and South Americas, three originate in Asia, and one comes from Africa. In the Americas, 28 species of this genus have been recorded, including 13 endemic species. In Taiwan, the only record of the genus was about Prosopis juliflora, found by Mr Yaiti Simada on the March 1, 1920, in Hengchun Township, Pingtung County (Specimens Database of Native Plants in Taiwan).
Chemical compounds in Prosopis spp. change certain physiological processes in the human body. Besides medicinal applications, different mesquite species have other uses. Since its wood is extremely hard and durable, and is of appealing coloration, it is used for making furniture and parquet flooring. Wood is also used for construction, as firewood, or for charcoal production [2]. Wooden chips provide mulch for gardening [3]. A beverage, known as “anapa,” is produced by mixing mesquite pods mixed in water After being fermented, it produces the alcoholic beverage “chichi” [4]. Owing to its high carbohydrate level, mesquite wood can also be used to produce bioethanol. Preliminary trials converted up to 80% of pod carbohydrates into bioethanol [3]. The aerial parts of Prosopis spp. from Argentina (Prosopis alpataco, Prosopis argentina, and Prosopis chilensis) demonstrated binding properties for DNA due to certain alkaloids. These also inhibited the antioxidant activity of β-glucosidase [5]. Medicinal values of Prosopis have been mentioned in ancient literatures [6].
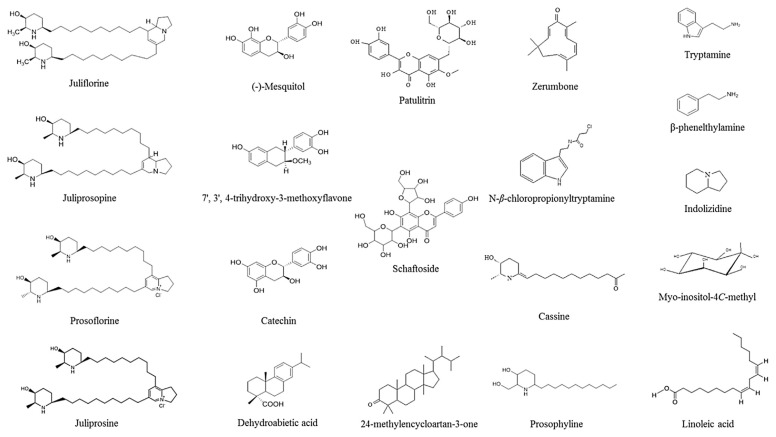
An early report by Kirtikar et al [7] mentioned that all parts of Prosopis spp. are traditionally used by indigenous people for curing various ailments [8]. Water extracts of leaves and bark are traditionally used to cure mouth and throat infections, as well as bronchitis and ulcers; internal diseases including parasites and urinary diseases; and skin parasitic infections as well as dermatitis [9]. The Indian Council of Forestry Research and Education (ICFRE) reported as early as 1993 that in Asia, medicinal uses of native Prosopis species included their flowers for the prevention of miscarriage and bark extracts for the treatment of bronchitis, leucoderma, tremors, asthma, rheumatism, leprosy, and dysentery. Leaf smoke is traditionally used to cure eye infections and extracts are recommended for use against snakebite and scorpion sting [10]. Ahmad et al [11] studied the antibacterial efficiency of juliflorine and julifloricine (structures of all mentioned phytochemicals are listed in Figure 1), as well as a benzene-insoluble alkaloid fraction of P. juliflora. Similarly, P. juliflora was reported with low mean antigiardiasic activity [12]. More recently, representatives of Prosopis were studied more intensively. These studies came along with advanced scientific technologies that demonstrated several medicinal properties of these species, such as antioxidant hepatoprotective, hemolytic, anticancer, antibacterial, antifungal, antidiabetic, and anti-inflammatory activities [13].
Figure 1.
Chemical structures of phytochemicals with medicinal properties present in Prosopis spp.
Alkaloids, flavonoids, terpenes, and phenolic compounds are the most important bioactive substances of Prosopis spp. [14]. Terpenes are used as insecticides, and their pharmacological properties include antibacterial, antifungal, antihelminthic, antimalarial, and molluscicidal activities [14]. Phenolic compounds from mesquite show anti-inflammatory, antitumor, anti-HIV, anti-infective, vasodilatory, antiulcerogenic analgesic, and immunostimulant activities [15]. Flavonoids have attracted interest, recently, due to the discovery of their pharmacological activities [16]. Alkaloids from mesquite are applied as analgesics and antimalarial agents. Alkaloids of Prosopis spp. also demonstrate a broad spectrum of antifungal activities against fungi such as Fusarium, Drechslera, and Alternaria. Flavonol glycosides and hydroxycinnamic acids from the pollens of P. juliflora provide antioxidants with high free radical scavenging activity [17,18]. Examples of these bioactive compounds are 3-oxo-juliprosopine and secojuliprosopinal isolated from P. juliflora [19].
Low production costs of a large amount of raw materials and straightforward integration into traditional agricultural practice provide certain advantages in the use of mesquite. This makes these plants particularly attractive in developing countries. However, the scientific validation of traditional forms of medicinal usage is still missing [20]. Most investigations of traditional medicinal applications are restricted to crude extracts with different solvents from a limited selection of representatives of Prosopis spp., with no further investigation on their bioactive compounds.
The present review summarizes the pharmaceutical potential of Prosopis spp. with respect to different diseases.
2. Biology of mesquite trees belonging to Prosopis spp
Mesquite trees have a deep-growing taproot that can even reach water tables at a depth of more than 30 m [21]. Mesquite trees exhibit species-specific differences and have a wide range of varieties. The most frequently occurring P. juliflora has a twisted stem with branches armed with strong thorns. Its leaves are bipinnate, the pale yellow flowers are arranged in spikes, it has flattened fruits with a solid epicarp, and the curved, pulpy mesocarp is sweet with several seeds [2,3].
P. juliflora is adapted to warm and dry tropical climates. It can grow in areas with an annual rainfall of only 250–600 mm. It is fast growing with a well-meshed deep-growing root system, as studied by Yoda et al [22]. P. juliflora can grow in extreme situations such as in rocky and saline soils. It is adapted to drought, and its leaves are avoided by most herbivores in agriculture. It is suitable for reforesting wastelands [23]. Some authors claim that P. juliflora also provides important ecosystem services related to the soil physical structure and nutrient cycling, compared with conventional plants in conventional monoculture. It recovers after enduring frost and moderate fire. It is claimed that after sprouting back in early spring, P. juliflora would regain its original size within less than 1 year [24].
Several Prosopis species can endure high temperatures and low rainfall, as well as saline, infertile, and even alkaline soils [3,25]. Whereas other congeners fail to withstand prolonged drought periods, P. juliflora endures those harsh times. Its seedlings survive drought by protecting young leaves with their folded cotyledons.
3. Medicinal applications
3.1. Antibacterial activity
Microbial antibiotic resistance is a matter of increasing concern. This calls for novel approaches in obtaining antimicrobial activity to treat infectious diseases in humans as well as in agricultural animals and plants. According to Ahmad et al [11], the compound juliflorine, which is generally synthesized within the genus Prosopis, provides protection against some human pathogenic bacteria, such as Corynebacterium diphtheria var. mitis, Corynebacterium hofmanni, Bacillus subtilis, Staphylococcus aureus, and Streptococcus pyogenes (see Table 1). Interestingly, juliflorine offered substantial antibiotic efficacy even against Streptococcus faecalis, which is resistant to most antibiotics [26,27]. Compared with the antibiotics streptomycin and penicillin, ethanol extracts of Prosopis spp. provide antibacterial activity against various pathogenic bacteria (see Table 1) [28]. Among human pathogenic bacteria, Pseudomonas aeruginosa provides one of the most threatening multidrug-resistant microbes, which has a multidrug efflux system containing at least 10 separated efflux pump system genes [29]. The minimal inhibitory concentrations of several unrelated antibiotics are increased by this efflux pump mechanism [30,31]. The crude methanol extracts of P. juliflora showed antibacterial activity against S. aureus and Escherichia coli [32]. The compounds myoinositol-4C-methyl and N-β-chloropropionyltryptamine (Figure 1) are particularly responsible for this activity [33]. In comparison to extracts of other plant parts, the extract of P. juliflora pods shows higher activity against Gram-positive bacteria, such as S. aureus [34].
Table 1.
Traditional usage of Prosopis spp. in herbal medicine.
| Species | Parts used | Treatment | Reference |
|---|---|---|---|
| P. cineraria | Leaf smoke Leaf extract Gum |
Treatment for eye infection, snakebite, and scorpion sting | [10] |
| P. cineraria | Flower | Safeguarding pregnant women against miscarriage | [89] |
| P. cineraria | Pods | Efficacious in rheumatism | [90] |
| P. juliflora | Leaf juice | Folk remedies for cancerous conditions | [91] |
| P. juliflora | Pods and Leaves | Remedy for catarrh cold, diarrhea, dysentery, excrescences, eye infections, flu, head cold, hoarseness, inflammation, itch, measles, pink eye, stomachache, sore throat, and wounds | [92] |
| P. juliflora | Leaf tea | Remedy for sore throat | [93] |
| P. grandulosa | Paste of leaves and fruit | Relieving pain associated with bone fracture in animals | [94] |
Different parts of P. juliflora provide different alkaloids, tannins, phenols, flavonoids, terpenes, and steroids [35,36]. Furthermore, ethanolic leaf and root extracts of P. juliflora showed antibacterial abilities against Gram-negative bacteria that were otherwise resistant to antibiotics such as minocycline, chloramphenicol, and erythromycin [37]. It was also noted that alkaloids, saponins, and tannins were likely candidates for this activity [38]. Plant diseases caused by the bacteria Agrobacterium rhizogenes and Xanthomonas campestris were shown to be cured by aqueous extracts of P. juliflora [39].
3.2. Antifungal activity
A severe deadly fungal disease harmful to patients infected by HIV/AIDS is caused by the fungal species Cryptococcus meningitis. Cryptococcus neoformans and Cryptococcus gattii are also involved [40]. Alkaloid-rich fractions of P. juliflora leaves inhibit the growth of C. neoformans, and the compounds zerumbone and cassine (Figure 1) exert high antifungal activity against this fungal species [41]. Plants’ defense mechanisms vary depending on the fungal pathogens they face [42]. Methanol extracts of P. juliflora leaves have substantial anti-fungal activity against mycelial growth of the fungus Colletotrichum musae, causing the most serious postharvested fungal disease in banana [43]. As shown by other experiments, the methanol extract of leaves is thermostable, showing tolerance of plant extracts to higher temperatures. This fraction shows considerable antifungal activity against seed-borne fungal pathogens, such as Aspergillus candidus, Aspergillus columnaris, Aspergillus flavipes, Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus ochraceus, and Aspergillus tamarii. Methanol and aqueous extracts of P. juliflora also show anti-fungal activities, especially against A. niger [44,45] and A. fumigatus [39], respectively. A study of tobacco plant infections caused by the fungal pathogen Alternaria alternata demonstrated the antifungal efficacy of P. juliflora leaf extracts [46]. Aqueous extracts of P. juliflora showed higher activity than synthetic fungicides.
3.3. Antiprotozoal activity
Eukaryotic protist infections are causing serious impacts on people, especially immune-compromised people, worldwide [47]. Methanol extracts of P. juliflora have high antiplasmodial activity against the malaria-causing protist Plasmodium falciparum and also antiflagellate activity against the vector of the Chagas disease, Trypanosoma cruzi [48]. Petroleum-ether extracts of P. juliflora leaves were assessed for intestinal, performing antigiardial, and amoebicidal activities and activity against pathogenic protists [49]. A study demonstrated high activity against Entamoeba histolytica, with 71.97% mortality after 72-hour exposure to the extract at a concentration of 1000 ppm. The study also showed high antibiotic activity against the protist Giardia lambila at 500 ppm.
3.4. Antioxidant potentials of Prosopis spp
Alkaloids from Prosopis spp. have a strong ability to capture free oxygen radicals [5]. Free radicals cause oxidative damage. Several other studies confirmed that P. juliflora have substantial antioxidant potential [28]. According to the research of Lakshmibai et al [50], ethanol extracts of P. juliflora leaves containing phytochemical alkaloids were able to scavenge free radicals. Siahpoosh and Mehrpeyma [51] demonstrated that polyphenol compounds from the bark of P. juliflora showed considerable dose-dependent antioxidant and free radical scavenging activities [45].
3.5. Anti-inflammatory activity
Anti-inflammatory activity of P. juliflora was demonstrated by carrageenan- and histamine-induced paw edema in rats [19]. Carrageenan is an organic nitrogenous compound (polysaccharide and histamine) used by Sivakumar et al [19] to induce inflammation in rats. Assays of carrageenan-induced paw edema and second histamine-induced paw edema indicated potent inhibition of inflammation by P. juliflora. Involvement of prostaglandins in the second phase of inflammation makes the formulation of anti-inflammatory drugs challenging; however, according to the experiments of Sivakumar et al [19], methanol extracts of P. juliflora bark inhibited carrageen-induced inflammation in rats by blocking prostaglandins. Moreover, ethanol extracts of the leaves of P. juliflora have substantial anti-inflammatory potency [52].
3.6. Oral disinfection
To overcome oral and periodontal infections, aqueous extracts of P. juliflora leaves can be used to eradicate bacterial infections from the periodontal space and oral cavity. Its efficacy is comparable with that of commercial mouth rinse containing synthetic compounds such as chlorhexidine gluconate, sorbitol, alcohol, n-propanol, eucalyptol, methyl salicylate, thymol, and sodium benzoate [53].
3.7. Antipustule activity
In patients with inflammatory skin disorders, such as atopic dermatitis, increasing severity of the disease strongly correlates with a decrease in microbial diversity and an increase in staphylococci. Metabolites of P. juliflora also possess anti-pustule activity. Acetone extracts of leaves, for example, can inhibit the growth of Staphylococcus sp. effectively by providing antipustule plant metabolites [54].
3.8. Antiulcer activity
Compounds such as alkaloids, flavonoids, tannins, anthraquinones, and quinones from P. juliflora have been explored as therapeutic drugs against ulcer. They can inhibit the growth of the ulcer-causing bacterium Helicobacter pylori through a mechanism where H+, K+, and ATPase are inhibited in combination with antioxidant activity. Ethanol extracts of P. juliflora exhibit a higher potential for ulcer reduction in rats than the normally available drug ranitidine [55]. Gobinath et al [56] showed the safe antipyretic activity of P. juliflora crude ethanol extracts.
3.9. Antiemetic activity
Crude methanol extracts of the leaves of Prosopis cineraria and P. juliflora (as well as other plant family representatives Adenanthera pavonina and Peltoforum roxburghii) were evaluated for their antiemetic activity in a study by Hasan et al [57]. The authors assayed the antiemetic activity by calculating the mean decrease in the number of reverse movements of the stomach and esophagus without vomiting. All extracts showed antiemetic activity when compared with the standard drug chlorpromazine at the same dose. Among all extracts, P. juliflora showed the highest antiemetic activity [57].
3.10. Role in antidiabetic activity
P. juliflora contains 24-methylencycloartan-3-one (Figure 1), which can safely be used to treat diabetes mellitus instead of using insulin [58]. This compound is contained in the oil of P. juliflora pods. Additionally, it has a good hypoglycemic effect in the screening assay of alloxan inducing fasted diabetic rabbits. The compound 24-methylencycloartan-3-one shows no cytotoxicity to red mammalian blood cells [6].
3.11. P. juliflora in cancer prevention and therapy
For the In vitro antitumor potentials, total alkaloid extractions from leaves of P. juliflora contain higher concentration-dependent cytotoxic effects on cancer cells than those of normal cells. This was indicated by effective cytotoxic activity against human T-cell leukemia cells [59].
Compounds from flowers of P. juliflora show anti-proliferative activity against mitotic cell divisions via chromosome aberrations. Antiproliferative activity is demonstrated by the root cells of the onion plant Allium cepa. A new compound found in 2012 from P. juliflora flowers provides a good spindle inhibitor which can proceed the clastogenic effects in A. cepa cells [60]. This compound induces different mutations such as chromosomal aberrations, fragmentations, and C-mitotic effects.
3.12. P. juliflora in Alzheimer therapy
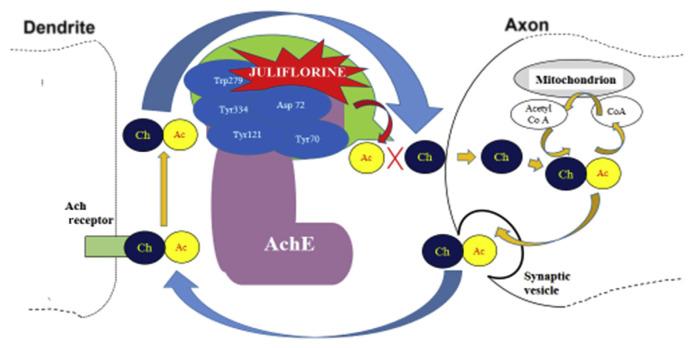
Alzheimer disease is a neurodegenerative disorder, such as dementia, which most occurs in older people. Cholinergic brain synapses and neuromuscular junctions are affected. The World Health Organization declared dementia as a priority condition through the Mental Health Gap Action Program in 2008 [61]. Acetyl cholinesterase plays an important role in the functioning of neuronal synapses. Acetyl cholinesterase hydrolyzes cationic neurotransmitters that terminate impulse transmission [62,63] (Figure 2).
Figure 2.
Mechanisms of action of juliflorine from Prosopis spp.
P. juliflora contains specific alkaloids that affect neuro-degenerative diseases. The alkaloid juliflorine from P. juliflora can inhibit acetyl cholinesterase in a concentration-dependent manner. Choudhary et al [64] proved that juliflorine plays a potent synergy to acetylcholinesterase by producing different forces such as hydrophobic contacts and hydrogen bond between juliflorine and amino acid residues of the aromatic gorge in acetylcholinesterase (Figure 2). Amino acid residues, in particular Tyr70, Asp72, Tyr121, Trp279, and Tyr334, belonging to the anionic and peripheral sites of acetylcholinesterase are involved. Juliflorine also showed dose-dependent spasmolytic and Ca2+ channel-blocking activity in jejunum preparations of rabbits [63].
4. Veterinary medical applications
4.1. Anthelmintic activity
Nematode infections of the gastrointestinum cause mortality, loss of nutrient absorbance efficiency, weakness, and retarded growth in ruminant livestock. Even the death of animals due to nematode infections is common in tropical regions where control programs are solely based on the use of synthetic anthelmintica. These are of high costs to poor farmers and of concern with respect to the presence of residues in food and the environment. Alternative control methods are thus required. Analysis of nematode egg hatching assays demonstrated striking differences in the bioactivities of the ethanol extracts [65] of P. juliflora roots and leaves.
The anthelmintics with an effective potential for livestock are provided by aqueous leaf extracts of P. juliflora and roots of Entada leptostachya powder. The herbal drugs mixture contains compounds as alkaloids, steroids, phenolic compounds, tannins, flavonoids, and saponins [66]. This could provide an alternative to the use of chemical drugs for deworming applications. The encapsulated ethanolic extracts of P. juliflora leaves display ovicidal potential against Haemonchus contortus, a highly pathogenic nematode parasite of small ruminants [67]. The leaves inhibit egg hatching of H. contortus. Nematode egg hatching inhibition may offer a suitable bioassay for the estimation of compound efficacies.
5. Indirect medicinal effects of P. juliflora
5.1. Antimosquito activity
Mosquito control by synthetic insecticides is creating environmental problems worldwide. Larvicidal activity of acetone extracts of P. juliflora leaves provide substantial mortality against Anopheles stephensi mosquito larvae [68]. Representatives of the genus Anopheles are major vectors of malaria. Its application would provide an ecofriendly approach to mosquito control. Along with its larvicidal activity, it also shows considerable activity against the adults of the mosquito A. stephensi [69].
5.2. Biomedical applications of products associated with or processed from P. juliflora
A high amount of antimicrobial compounds from P. juliflora could be mainly due to the capability of fungal endophytes to provide benefits to its host plant [70]. A fungal endophyte from the leaves of P. juliflora shows antimicrobial potential against plant and human pathogenic bacteria and fungi. The ethyl acetate extracts of fungal endophytes isolated from the leaves of P. juliflora reveal activity against pathogenic bacteria and other fungi [71]. The endophyte Paecilomyces lilacinus, for example, exerts significant antifungal activity against the fungus Colletotrichum gloeosporioides, which in turn shows antibacterial activity.
5.3. Monofloral honey and pollens from “mesquite”
Ethanol crude extracts of honeybee-collected pollens of P. juliflora have significant inhibitory activity against lipid peroxidation. The flavonols present in monospecific mesquite honeybee-collected pollens are at least partly responsible for its antioxidant capacity. It provides inhibition against lipid peroxidation in rodents [71].
5.4. Medicinal substances of cyanobacteria-degraded products of P. juliflora
Ethanol extracts of P. juliflora degraded by the cyanobacterium Oscilatoria laetevirens, with abundant alkaloids, flavonoids, terpenoids, and steroids, provide potent free oxygen radical scavenging activity [72]. P. juliflora extracts also provide anti-oxidants that include alphatocopherol and probucol (Figure 1). Probucol shows considerable antioxidant activity against inflammatory diseases. Degraded ethanol extracts of P. juliflora can have considerable potential for pharmacological applications [73].
5.5. Mucilage of P. juliflora as tablet binder
The hydrophilic mucilage from the seeds of P. juliflora was studied for its potential of mucilage binding in tablet formulations [74]. In this study, granules were prepared by a wet granulation technique. The granules had excellent flow properties. Tablets prepared using 8% and 10% of mucilage were harder than other formulations and showed drug release over a period of 5 hours.
5.6. Probiotic and nutritional values
Mesquite pods have a high nutritional value. Astudillo et al [75] described the pod composition of different mesquite representatives from Chile as rather variable. In their study, Prosopis spp. contained about 16–41% of total sugars, 10–15% DM (dry matter) protein, and 20–30% DM crude fiber. Fresh leaves contain about 17–20% protein and 22% crude fiber. They are rich in lysine but deficient in methionine and cysteine. The hay contains about 14% protein. Seeds contain up to 30–40% protein and much less fiber (3–7%) than the pods. Pod husks are rich in crude fiber (54% DM) and poor in protein (4% DM) [75]. Pods from India and Africa have less carbohydrate but more fiber than pods from Brazil and Peru. The sugar content makes them palatable to animals in the agricultural industry.
6. Conclusion
The plant genus Prosopis has important applications in medicinal products for human use as well as in veterinary medicine (Table 2), include antidiabetic, anti-inflammatory, anticancer, and antimicrobial activities. Compounds from this taxon demonstrate antibiotic activity against microbial pathogens and enzyme inhibition activities, and show potential in other pharmaceutical applications, such as binding abilities for tablet production, inhibition of H+, K+, ATPase of H. pylori, and acetylcholine esterase inhibiting activity. Especially, the components, flavonoids, tannins, alkaloids, quinones, or phenolic compounds, demonstrate potentials in various biofunctions.
Table 2.
Medicinal effects of bioactive substances from parts of the Prosopis spp (Leguminosae, Mimosaceae).
| Prosopis species | Parts | Medicinal effects | Bioactive components | References |
|---|---|---|---|---|
| P. juliflora | Leaves | Inhibition of acetyl cholinesterase Blocking calcium channels | Juliflorine | [64] |
| Leaves, pods | Decreasing gas production during ruminal digestion | Juliprosopine Prosoflorine Juliprosine |
[76] | |
| Fruits | Activity against lung carcinoma | Patulitrin | [77] | |
| Heart wood | Antioxidant activity | (−)-Mesquitol | [78] | |
| Leaves | Inhibition of drug-resistant fungi | Piperidine alkaloids | [41] | |
| Glial cell activation | Alkaloids | [79] | ||
| Seeds, leaves | Inhibition of H+, K+, ATPase of H. pylori | Alkaloids, flavonoids, tannins, anthraquinones, and quinones | [56] | |
| P. cineraria | Pods | Antioxidant activity | Triterpenoids Prosophyline |
[80] |
| Cyclooxygenase enzyme inhibition activity | Linoleic acid | |||
| Prosopis africana | Leaves, roots and Stem | Antitripanosomal activity | Tannins | [81] |
| Stem bark | Anti-inflammatory activity | Flavonoids | [75] | |
| Stem bark | Antibacterial activity (Mycobacterium aurum, S. aureus) Breast cancer cell line inhibition |
7′, 3′, 4-Trihydroxy-3-methoxyflavone Dehydroabietic acid |
[82] | |
| P. chilensis | Leaves | DNA binding activity | β-phenethylamine Tryptamine |
[75] |
| Prosopis glandulosa var. glandulosa | Leaves | Anti-infective Antiparasitic Antiparasitic Antimicrobial activity |
Indolizidine Juliprosopine |
[83] |
| Prosopis flexuosa | Aerial parts | Antioxidant activity | Catechin | [5] |
| Prosopis tamarugo | Leaves | [75] | ||
| Prosopis alpha | Leaves, pods | [84] | ||
| Prosopis alba | Pod flour | Schaftoside | [85] | |
| Prosopis nigra | ||||
| P. alpataco | Seed | Antibacterial activity | Pentacyclic triterpenes | [86] |
| Prosopis denudans | ||||
| Prosopis pallida | Fruit | Antihyperglycemia | Phenolic compound | [87] |
| Antihypertension | ||||
| P. cineraria | Whole plant | Anticonvulsant activity | Alkaloids | [88] |
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Nadkarni KM, Nadkarni AK. Dr K.M Nadkarni’s Indian materia medica: with Ayurvedic, Unani-tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes. Bombay: Popular Prakashan; 1976. [Google Scholar]
- 2. Mwangi E, Swallow B. Prosopis juliflora invasion and rural livelihoods in the lake Baringo area of Kenya. Conserv Soc. 2008;2:130–40. [Google Scholar]
- 3.Pasiecznik NM, Felker P, Harris PJC, Harsh LN, Cruz G, Tewari JC, Maldonado LJ. The Prosopis juliflora–Prosopis pallida complex: a monograph. Coventry, UK: HDRA; 2001. [Google Scholar]
- 4. Vilela A, Bolkovic ML, Carmanchahi P, Cony M, de Lamo D. Past, present and potential uses of native flora and wildlife of the Monte Desert Wassnerg. J Arid Environ. 2009;73:238–43. [Google Scholar]
- 5. Tapia A, Egly Feresin G, Bustos D, Astudillo L, Theoduloz C, Schmeda-Hirschmann G. Biologically active alkaloids and a free radical scavenger from Prosopis species. J Ethnopharmacol. 2000;71:241–6. doi: 10.1016/s0378-8741(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 6. Rajvanshi S, Garg V. King of desert (Prosopis): a source of potential medicinal values in Arid Zones of India: review. Int J Res Eng Appl Sci. 2015;5:185–91. [Google Scholar]
- 7.Kirtikar K, Basu B, Blatter E, Kirtikar K, Basu B, Blatter E. Indian medicinal plants. 2nd ed. Allahabad, India: Lalit Mohan Basu; 1935. [Google Scholar]
- 8. Khejra CD. Khejra, Vanoshdi Chitravali (Jaributti) 2001;1:269–70. [Google Scholar]
- 9. Pasiecznik NM. Prosopis: pest or providence, weed or wonder tree? Eur Trop For Res Netw Newslett. 1999;28:12–4. [Google Scholar]
- 10. Pimental MDL. P.juliflora (SW)(DC) 1 “Simposio Brasilerio sobre Algarobera”. 1960;1:330–5. [Google Scholar]
- 11. Ahmad VU, Sultana A, Qazi S. Alkaloids from the leaves of Prosopis juliflora. J Nat Prod. 1989;52:497–501. [Google Scholar]
- 12. Ponce-Macotela M, Navarro-Alegría I, Martínez-Gordillo MN, Alvarez C. Effect to antigiardiásico in vitro de 14 extractos de plantas. Rev Invest Clin. 1994;46:343–7. [PubMed] [Google Scholar]
- 13.Duke JA, Boganschutz-Godwin MJ, Ducelliar J, Duke PK. Hand book of medicinal herbs. 2nd ed. Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- 14. Gurib-Fakim A. Medicinal plants: tradition of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15. Stefanović OD, Tešić JD, Čomić LR. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal. 2015;23:417–24. doi: 10.1016/j.jfda.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lou SN, Hsu YS, Ho CT. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J Food Drug Anal. 2014;22:290–5. doi: 10.1016/j.jfda.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wierman R, Vieth K. Outer pollen wall, an important accumulator site for flavonoids. Protoplasma. 1983;118:230–3. [Google Scholar]
- 18. Campos MG, Markham KR, Proenc da Cunha A. Quality assessment of bee-pollens using flavonoid, phenolic profiles. Polyph Comm. 1997;96:53–4. [Google Scholar]
- 19. Sivakumar T, Srinivsan K, Rajavel R, Vasudeva M, Ganesh M, Kamalakannan K, Mallika P. Isolation of chemical constituents from Prosopis juliflora bark and anti-inflammatory activity of its methanol extracts. J Pharm Res. 2009;2:551–6. [Google Scholar]
- 20.Abbott R. Documenting traditional medical knowledge. Geneva, Switzerland: WIPO; 2014. [Google Scholar]
- 21. Riveros F. The genus Prosopis and its potential to improve livestock production. Legume trees and other fodder trees as protein sources for livestock. Ann Bot. 1992;70:399–404. [Google Scholar]
- 22. Yoda K, Elbasti MA, Hoshino B, Nawata H, Yasuda H. Root system development of Prosopis seedlings under different soil moisture conditions. J Arid Land Stud. 2012;22:13–6. [Google Scholar]
- 23. Vallejo VE, Arbeli Z, Terán W, Lorenz N, Dick RP, Roldan F. Effect of land management and Prosopis juliflora (Sw.) DC trees on soil microbial community and enzymatic activities in intensive silvo pastoral systems of Colombia. Agric Ecosyst Environ. 2012;150:139–48. [Google Scholar]
- 24. Saxena SK, Khan WA. A quick method for obtaining clean seeds of Prosopis juliflora. Ann Arid Zone. 1974;13:269–72. [Google Scholar]
- 25. Shiferaw H, Teketay D, Nemomissa S, Assefa F. Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) DC at Middle Awash Rift Valley Area, Northeastern Ethiopia. J Arid Environ. 2004;58:135–54. [Google Scholar]
- 26. Criswell D. The “evolution” of antibiotic resistance. Impact: Vital Artic Sci/Creat. 2004;378:1–4. [Google Scholar]
- 27. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 28. Sathiya M, Muthuchelian K. Investigation of phytochemical profile and antibacterial potential of ethanolic leaf extracts of Prosopis juliflora DC. Ethnobot Leaflets. 2008;12:1240–5. [Google Scholar]
- 29. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 30. Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453:254–67. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 31. Elizabeth B, Tam V. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Exp Rev Pharmacoecon Outcomes. 2010;10:441–51. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sukirtha K, Growther L. Antibacterial, antifungal and phytochemical analysis of selected medicinal plants. J Nat Prod Plant Res. 2012;2:644–8. [Google Scholar]
- 33. Vedak S, Raut SV. Study on antibacterial compounds from methanolic extract of bark of Prosopis juliflora (Vilayati Babhul) Int J Pharm Sci Bus Manag. 2014;2:1–14. [Google Scholar]
- 34. Saheed T, Alireza B, Vakhshiteh F, Gharibi M. In vitro antibacterial activity of the Prosopis juliflora seed pods on some common pathogens. J Clin Diagn Res. 2015;9:DC13–5. doi: 10.7860/JCDR/2015/13549.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sing S. Phytochemical analysis of different parts of Prosopis juliflora. Int J Curr Pharm Res. 2012;4:59–61. [Google Scholar]
- 36. Singh S, Verma SSK. Antibacterial properties of alkaloid rich fractions obtained from various parts of Prosopis juliflora. Int J Pharm Pharm Sci. 2011;2:114–20. [Google Scholar]
- 37. Odhiambo RS, Patrick KG, Helen KL, Gathu NC, Kimani NF, Waithaka WR, Kipyegon C. Antibacterial activity of ethanolic extracts of Prosopis juliflora against Gram negative bacteria. Eur J Exp Biol. 2015;5:43–6. [Google Scholar]
- 38. Thakur R, Singh R, Saxena P, Abin M. Evaluation of antibacterial activity of Prosopis juliflora (SW). DC. leaves. Afr J Trad Complement Altern Med. 2014;11:182–8. doi: 10.4314/ajtcam.v11i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheikh M, Malik AR, Meghavanshi MK, Mahmood I. Studies on some plant extracts for their antimicrobial potential against certain pathogenic microorganisms. Am J Plant Sci. 2012;3:209–13. [Google Scholar]
- 40. Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2005;72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 41. Valli S, Gokulshankar S, Mohanty BK, Ranjit MS, Ashutosh SR, Remya V. Antistreptococcal activity of alkaloid rich fraction of leaves of Prosopis juliflora—a future promising supplementary therapy for cryptococcosis and cryptococcal meningitis. Int J Pharm Pharmacol Sci. 2014;6:490–5. [Google Scholar]
- 42. Dehgahi R, Subramaniam S, Zakaria L, Joniyas A, Firouzjahi F, Haghnama K, Razinataj M. Review of research on fungal pathogen attack and plant defense mechanism against pathogen. Int J Sci Res Agric Sci. 2015;2:197–208. [Google Scholar]
- 43. Bazie S, Ayalew A, Woldetsadik K. Antifungal activity of some plant extracts against Colletotrichum musae the cause of postharvest banana anthracnose. J Plant Path Microb. 2014;5:1–4. [Google Scholar]
- 44. Satish S, Mohana DC, Raghavendra MP, Raveesha KA. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp J Agric Sci Technol. 2007;1:109–19. [Google Scholar]
- 45. Napar AA, Bux H, Zia MA, Ahmad MZ, Iqbal A, Roomi S, Muhammad I, Shah SH. Antimicrobial and antioxidant activities of Mimosaceae plants; Acacia modesta Wall (Phulai), Prosopis cineraria (Linn.) and Prosopis juliflora (Swartz) J Med Plant Res. 2012;6:2962–70. [Google Scholar]
- 46. Raghavendra MP, Satish S, Raveesha KA. Alkaloids isolated from leaves of Prosopis juliflora against Xanthomonas pathovars. Arch Phytopathol Plant Prot. 2009;42:1033–41. [Google Scholar]
- 47. Barratt J, Harkness D, Marriott JT, Ellis JT, Stark D. Importance of nonenteric protozoan infections in immunocompromised people. Clin Microb Rev. 2010;23:795–836. doi: 10.1128/CMR.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Musayeib NM, Mothana RA, Al-Massarani S, Matheeussen A, Cos P, Maes L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules. 2012;17:11379–90. doi: 10.3390/molecules171011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garbi MI, Osman EE, Dahab MM, Koko WS, Kabbashi AS, Elegami nad Sheeema AA, Hameed Y. Antigiardial, amoebicidal and cytotoxic activity of the plant Prosopis juliflora leave extracts. Res J Biochem. 2014;2:1–7. [Google Scholar]
- 50. Lakshmibai R, Amirtham D, Radhika S. Preliminary phytochemical analysis and antioxidant activities of Prosopis juliflora and Mimosa pudica leaves. Int J Sci Eng Technol Res. 2015;4:5766–70. [Google Scholar]
- 51. Siahpoosh A, Mehrpeyma M. Antioxidant effects of Albizia lebbek and Prosopis julilfora barks. Int J Biosci. 2014;5:273–84. [Google Scholar]
- 52. Choudhary PK, Nagori BP. Oral Prosopis juliflora treatment ameliorates inflammatory responses against carrageenan induced paw edema in rats. World J Pharm Sci. 2013;2:5718–24. [Google Scholar]
- 53. Hari Prasad O, Aluru S, Kishore Kumar A, Navya A, Hari Krishna O, Bhaskar M, Papa Rao A, Reddy NR. Comparative evaluation of the antibacterial efficacy of P. juliflora and three commercially available mouthrinses: an in vitro study. J Pharm Res. 2011;4:2149–51. [Google Scholar]
- 54. Jesudoss RP. Screening of anti-pustule plant metabolites from Prosopis juliflora and their combined antipustule activity with synthetic pimple creams. J Chem Pharm Sci. 2014;2:145–50. [Google Scholar]
- 55. Jagan Mohan Reddy P, Tejaswini DM, Ismail Shareef M, Narasimha Murthy TP, Gopinath SM. Evaluation of antiulcer activity of Prosopis juliflora ethanol, extract in ethanol induced gastric ulceration in rats. Int J Pharm Rev Res. 2014;41:17–20. [Google Scholar]
- 56. Gobinath SM, Reddy KS, Shankar T. To evaluate the antipyretic activity of Prosopis juliflora ethanolic extract in Brewer’s yeast induced hyperthermia in rats. J Biotech Biosaf. 2013;1:28–32. [Google Scholar]
- 57. Hasan MMU, Azhar I, Muzammil S, Ahmed S, Ahmed SW. Anti-emetic activity of some leguminous plants. Pak J Bot. 2012;44:389–91. [Google Scholar]
- 58. Alsaadi JHH, Al-Maliki ADM. Hypoglycemic effect of 24-methylencycloartan-3-one isolated from Prosopis juliflora pods in alloxan induced diabetic rabbits. World J Exp Biosci. 2015;1:6–13. [Google Scholar]
- 59. Sathiya M, Muthuchelian K. Anti-tumor potential of total alkaloid extract of Prosopis juliflora DC. leaves against Molt-4 cells in vitro. Afr J Biotechnol. 2011;10:8881–8. [Google Scholar]
- 60. Shachi S. Antimitotic activity of a new compound isolated from the flower of Prosopis juliflora. Res J Recent Sci. 2012;1:22–6. [Google Scholar]
- 61. Korolev IO. Alzheimer’s disease: a clinical and basic science review. MSRJ. 2014;4:24–33. [Google Scholar]
- 62. Tougu V. Acetylcholinesterase: mechanism of catalysis and inhibition. Curr Med Chem Cent Nerv Syst Agents Chem. 2001;1:155–70. [Google Scholar]
- 63. Soreq H, Seidman S. Acetylcholinesterase-new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 64. Choudhary MI, Nawaz SA, Azim MK, Ghayur MN, Lodhi MA, Jalil S, Khalid A, Ahmed A, Rode BM, Ahmad VU. Juliflorine: a potent natural peripheral anionic-site-binding inhibitor of acetylcholinesterase with calcium-channel blocking potential, a leading candidate for Alzheimer’s disease therapy. Biochem Biophys Res Comm. 2005;332:1171–9. doi: 10.1016/j.bbrc.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 65. Odhiambo RS, Kareru GP, Kutima LH, Nyagah CG, Njonge KF, Waithaka WR. Evaluation of in-vitro ovicidal activity of ethanolic extracts of Prosopis juliflora (Sw.) DC (Fabaceae) J Pharm Biol Sci. 2014;9:15–8. [Google Scholar]
- 66. Mutembei J, Kareru P, Njonge F, Peter G, Kutima H, Karanja J, Kimmani D. In vivo anthelmintic evaluation of a processed herbal drug from Enta leptostachya (Harms) and Prosopis juliflora (Sw). (DC) against gastrointestinal nematodes in sheep. J Polymer Textile Eng. 2015;2:6–10. [Google Scholar]
- 67. Kipyegon C, Helen KL, Patrick KG, Francis NK, Odhiambo RS, Jackson MK, Edwin M. In vitro ovicidal activity of encapsulated ethanolic extract of Prosopis juliflora against Haemonchus contortus eggs. J Pharm Biol Sci. 2015;10:18–22. [Google Scholar]
- 68. Varun T, Ruchi Y, Ajay Kumar SA, Tyagi V, Yadav S, Veer V, Devanathan S. Larvicidal activity of leaf extract of some weeds against malaria vector Anopheles stephensi. Int J Malaria Res Rev. 2013;1:35–9. [Google Scholar]
- 69. Senthilkumar N, Varma P, Gurusubramanian G. Larvicidal and adulticidal activities of some medicinal plants against the malarial vector, Anopheles stephensi (Liston) Parasitol Res. 2009;104:237–44. doi: 10.1007/s00436-008-1180-4. [DOI] [PubMed] [Google Scholar]
- 70. Strobel GA. Endophytes as sources of bioactive products. Microb Infect. 2003;5:535–44. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 71. Srivastava A, Anandrao RK. Antimicrobial potential of fungal endophytes isolated from leaves of Prosopis juliflora (DC). An important weed. Int J Pharm Pharmacol Sci. 2015;7:128–36. [Google Scholar]
- 72. Abarca AN, Camposc MG, Jose AA, Reyesa V, Jimeneza NN, Herrera Corrala J, Gonzalez-Valdeza LS. Antioxidant activity of polyphenolic extract of monofloral honeybee collected pollen from mesquite (Prosopis juliflora, Leguminosae) J Food Compost Anal. 2007;20:119–24. [Google Scholar]
- 73. Prabha DS, Dahms HU, Ananth S, Pazhanisamy S, Malliga P. Bioactive substances from Prosopis juliflora (Sw.) DC.—degraded by the cyanobacterium Oscillatoria laetevirens. J Basic Appl Biol. 2015;2:306–71. [Google Scholar]
- 74. Selvi RS, Gopalakrashanan S, Ramajayam M, Soman R. Evaluation of mucilage of Prosopis juliflora as tablet binder. Int J Pharm Pharm Sci. 2010;2:157–60. [Google Scholar]
- 75. Astudillo L, Schmeda-Hirschmann G, Herrera JP, Corte M. Proximate composition and biological activity of Chilean Prosopis species. Inst J Sci Food Agric. 2000;80:567–73. [Google Scholar]
- 76. Dos Santos ET, Pereira MLA, da Silva CFPG, Souza-Neta LC, Geris R, Martins D, Santana AEG, Barbosa LCA, Silva HGO, Freitas GC, Figueiredo MP, de Oliveira FF, Batista R. Antibacterial activity of the alkaloid enriched extract from Prosopis juliflora pods and its influence on in vitro ruminal digestion. Int J Mol Sci. 2013;14:8496–516. doi: 10.3390/ijms14048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sathiya M, Muthuchelian K. Evaluation of antioxidant and antitumor potentials of Prosopis juliflora DC. leaves in vitro. Pharmacol Online. 2010;2:328–43. [Google Scholar]
- 78. Sirmah PF, Buru M, Laych K, Dumarcay S, Gerardin P. Potential antioxidant compounds from different parts of Prosopis juliflora. J Trop For Sci. 2011;23:187–95. [Google Scholar]
- 79. Silva AM, Silva AR, Pinheiro AM, Freitas SR, Silva VD, Souza CS, Hughes JB, El-Bachá RS, Costa MF, Velozo ES, Tardy M, Costa SL. Alkaloids from Prosopis juliflora leaves induce glial activation, cytotoxicity and stimulate NO production. Toxicon. 2007;49:601–14. doi: 10.1016/j.toxicon.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 80. Liu Y, Singh D, Nair MG. Pods of khejri (Prosopis cineraria) consumed as a vegetable showed functional food properties. J Funct Foods. 2012;41:116–21. [Google Scholar]
- 81. Atawodi SE, Ogunbusola F. Evaluation of anti-trypanosomal properties of four extracts of leaves, stem and root barks of Prosopis africana in laboratory animals. Bikemistri. 2009;21:101–8. [Google Scholar]
- 82. Elmezughi J, Shittu H, Clements C, Edrada-Ebel RA, Seidel V, Gray A. Bioactive natural compounds from Prosopis africana and Abies nobili. J Appl Pharm Sci. 2013;3:40–3. [Google Scholar]
- 83. Rahman AA, Samoylenko V, Jacob MR, Sahu R, Jain SK, Khan SI, Tekwani BL, Muhammad I. Antiparasitic and antimicrobial indolizidines from the leaves of Prosopis glandulosa var. glandulosa. Planta Med. 2011;77:1639–43. doi: 10.1055/s-0030-1270906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ayanwuyi LO, Yaro AH, Abodunde OM. Analgesic and antiinflammatory effects of the methanol stem bark extract of Prosopis africana. Pharm Biol. 2010;48:296–9. doi: 10.3109/13880200903121006. [DOI] [PubMed] [Google Scholar]
- 85. Cattaneoa F, Costamagna MS, Zampini IC, Sayago J, Alberto MR, Chamorrod V, Pazos A, Thomas-Valdése S, Schmeda-Hirschmann G, Isla MI. Flour from Prosopis alba cotyledons: a natural source of nutrient and bioactive phytochemicals. Food Chem. 2016;11:89–96. doi: 10.1016/j.foodchem.2016.03.115. [DOI] [PubMed] [Google Scholar]
- 86. Mazzuca M, Kraus W, Balzaretti V. Evaluation of the biological activities of crude extracts from patagonian Prosopis seeds and some of their active principles. J Herb Pharmacother. 2003;3:31–7. [PubMed] [Google Scholar]
- 87. Pinto MDS, Ranilla LG, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Evaluation of antihyperglycemia and antihypertension potential of native Peruvian fruits using in vitro models. J Med Food. 2009;12:278–91. doi: 10.1089/jmf.2008.0113. [DOI] [PubMed] [Google Scholar]
- 88. Sachdeva S, Kaushik V, Saini V. A review on phytochemical and pharmacological potential of Prosopis cineraria. Int J Ethnobiol Ethnomed. 2014;1:1–4. [Google Scholar]
- 89.Pakrashi S, Chatterjee A. The treatise on Indian medicinal plants. Vol. 2. New Delhi, India: Publication and Information Directorate; 1992. p. 112. [Google Scholar]
- 90. Ukani MD, Limbani NB, Mehta BNK. Review on the Ayurvedic herb Prosopis cineraria (L) Druce. Anc Sci Life. 2000;1:58–70. [PMC free article] [PubMed] [Google Scholar]
- 91. Hartwell JL. Plants used against cancer. A survey. Lloydia. 1967;30:30–4. [PubMed] [Google Scholar]
- 92.Duke JA. Handbook of legumes of world economic importance. New York: Plenum Press; 1981. [Google Scholar]
- 93.Lewis WH, Elvin-Lewis MPF. Medical botany. New York: John Wiley & Sons; 1977. [Google Scholar]
- 94. Khan FM. Ethno-veterinary medicinal usage of flora of greater Cholistan desert (Pakistan) Pak Vet J. 2009;29:75–80. [Google Scholar]