Abstract
Cardiac strangulation is a rare but potentially life-threatening mechanical complication associated with epicardial pacemaker implantation in growing children. This article presents 2 case reports of left ventricular strangulation in 4- and 3-year-old children who had an epicardial pacemaker system implanted at an early age. (Level of Difficulty: Advanced.)
Key Words: cardiac pacing, complication, pediatric surgery
Abbreviations and Acronyms: CHB, complete heart block; CS, cardiac strangulation; DDDR, dual chamber rate adaptive pacing mode; ECG, electrocardiogram; LV, left ventricle; RA, right atrium
Central Illustration
Cardiac strangulation (CS) is a rare mechanical complication due to implanted epicardial pacing system in a growing child, causing compression of the heart or great vessels. It can lead to constriction of the coronary arteries, valve insufficiency, or ventricular dysfunction. depending on the area of maximum compression.1,2
Learning Objectives
-
•
To raise awareness of the complication development of epicardial pacing in children.
-
•
To discuss follow-up of children with implanted epicardial pacemakers at an early age.
Case Summary
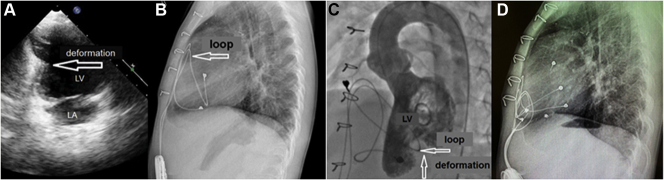
The first patient was 4 years of age. A dual-chamber (Boston Scientific ALTRUA 50 with leads CAPSURE EPI 4965) pacemaker with a unipolar lead was implanted on the 11th day after the surgery for ventricular septal defect complicated with complete heart block (CHB). Pacing mode was dual-chamber rate adaptive pacing mode (DDDR), pacing rate was 110/185 beats/min, and dynamic atrioventricular delay was 80/130 ms. Pacing thresholds were (A) 0.8 V and (V) 0.9 V. Amplitude was as follows: atrial, 3 V; and ventricular, 3 V. At age 4 years, Echo showed normal size of heart chambers, damaged left ventricle geometry (“hourglass head”) due to myocardial compression by an epicardial lead (Figure 1A).
Figure 1.
First Patient
(A) Echocardiography: left ventricle (LV) geometry is damaged (“hourglass head”). (B) Lateral view of the x-ray. The pacemaker lead was completely wrapped around the cardiac silhouette. (C) Left ventriculography showing deformation of LV contour in the apex view. (D) Lateral view of the postoperative x-ray showing the leads well placed at the bottom of the pericardium. LA = left atrium.
The lateral x-ray showed that the right atrium (RA) lead after looping left ventricle (LV) sutured to RA. The LV lead was located under the diaphragmatic heart surface (Figure 1B).
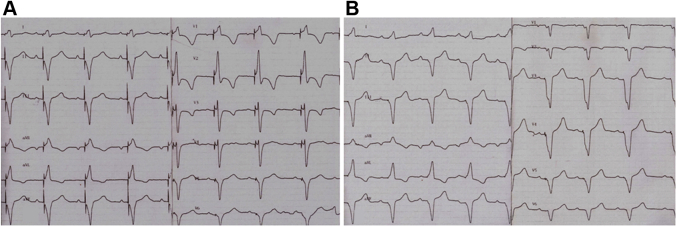
Based on angiography results, there were no signs of compression of the coronary arteries (Figure 1C). Electrocardiogram was normal (Figure 2). Considering left ventricular deformation, it was decided to replace the epicardial leads and the pacemaker.
Figure 2.
Electrocardiogram
(A) The first case. (B) The second case. Pacing in DDD mode. P = synchronized ventricular pacing.
The surgery was performed using bypass. The access was through a median resternotomy. The atrial lead loop in the pericardium formed a strangulation groove on the anterior LV and diaphragmatic right ventricle surfaces (Figure 3). First of all, the ventricular lead was removed providing adequate access to the anterior and diaphragmatic heart surfaces. The atrial lead was separated along its entire length and removed from the pericardial cavity. After epicardial lead extraction, the pacemaker was removed. Pacemaker parameters after reimplantation were as follows: intrinsic rhythm was <30 beats/min; pacing mode, DDDR; pacing rate, 70/200 beats/min; and atrioventricular delay, 150/120 milliseconds. RА lead was as follows: pacing threshold, 0.5 V; and amplitude, 1.5 V. Right ventricle lead was as follows: pacing threshold, 1.0 V; and amplitude, 2.25 V.
Figure 3.
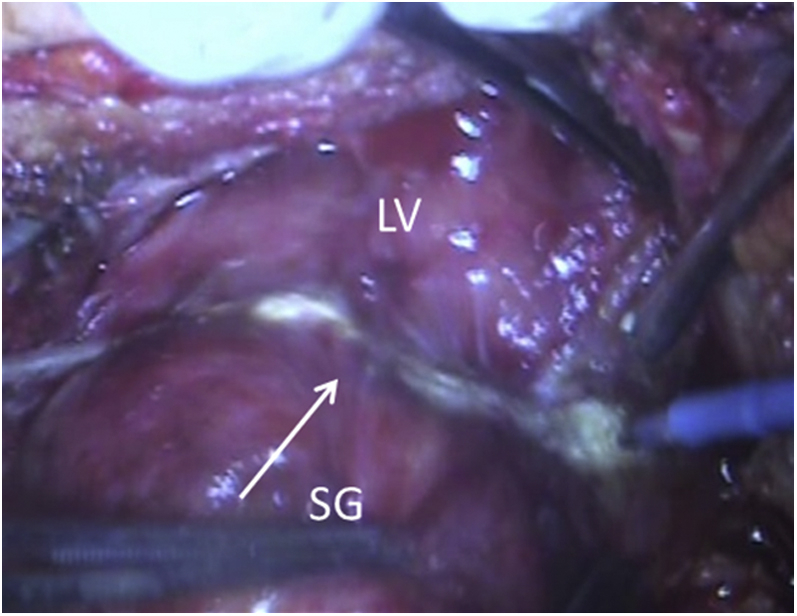
Strangulation Groove From the Loop of the Atrial Epicardial Lead
LV = left ventricle; SG = strangulation groove.
Considering the experience of this complication, we started to perform a chest x-ray in frontal and lateral views during follow-up in all patients with pacemakers implanted at an early age.
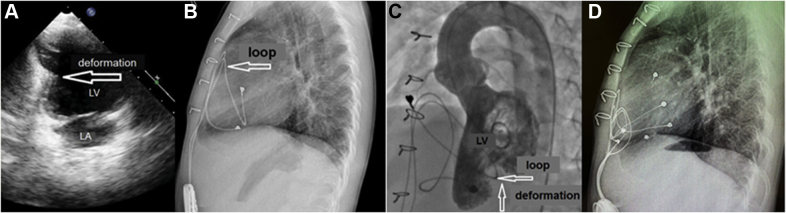
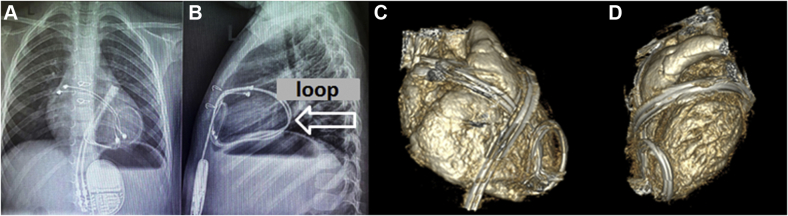
The next patient was a 3-year-old child. At age 1.5 years, a dual-chamber pacemaker was implanted due to congenital CHB. At initial implantation, his weight was 10 kg. At 6 months of follow-up his chest x-ray in frontal view showed no pathologic changes. Chest x-ray in lateral view was not performed. The next follow-up the patient underwent was at age 3.7 years. Electrocardiogram and (Figure 2) Echo were normal. Chest x-ray in lateral views showed the lead loop around the heart (Figures 4A and 4B). The child underwent chest computed tomography with intravenous bolus contrast that confirmed a loop around the heart. Conflicts with the structures of the heart and blood vessels were visually not detected (Figure 4C and 4D). Considering the absence of heart compression, we decided to refrain from urgent leads extraction. The child is currently under the close supervision of cardiologists.
Figure 4.
Second Patient
(A) Frontal view of the x-ray. (B) Lateral view of the c-ray confirming the diagnosis of cardiac strangulation showing a classic pattern of looping. (C) Computed tomography with intravenous bolus contrast confirming a loop around the heart; the conflicts with heart structures and vessels were not detected. The frontal view shows the epicardial leads looping left ventricle. (D) The lateral view shows the leads looping left ventricle.
Discussion
Only 15 CS cases in children after epicardial pacing have been reported (Table 1).2, 3, 4, 5, 6, 7, 8, 9 We added new case reports into the summarized table by Chihiro et al3 and specified the quantity of implanted leads and vital status age where data were available. In 2015, 2 author groups, Carreras et al4 and Takeuchi et al,2 concluded that CS incidence was much higher than it was presented in the literature.
Table 1.
Case Series of CS
| Case | Year | Location and Ref. # | Age at PMI | Age at CS | Primary Diagnosis | Number of Epicardial Leads | Compression | Symptom | Outcome Vital Status Age |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1988 | U.S.6 | 6 d | 20 mo | CAVB | 1 | RCA, PA, LMT | Syncope | Alive |
| 2 | 1992 | Japan4 | 3.1 y | 9.1 y | CAVB (TOF) | 2 | LCA (RCA?), PA | CHF | Unknown |
| 3 | 1997 | Belgium4 | 8 mo | 6 y | VSD | 2 | Apex | Chest pain | Death |
| 4 | 2000 | Japan4 | 2 d | 10 mo | CAVB | 1 | LCA, LCX | CHF (edema) | Death |
| 5 | 2000 | U.S.4 | 2 mo | 5 y | cJET | 1 | PA, "lasso" around the RVOT | New cardiac murmur due to pulmonary artery stenosis | Alive |
| 6 | 2007 | U.S.7 | <1 mo | 9 y | Significant bradycardia | 1 | LAD, LCX | Chest pain | Alive 10 y |
| 7 | 2007 | U.S.7 | 7 d | 12 y | CAVB | 1 | LCA, LV | None | Alive |
| 8 | 2008 | Germany4 | 3 mo | 2.7 y | CAVB | 2 | LCA | CHF (dilated cardiomyopathy) | Alive 6 y |
| 9 | 2011 | Canada4 | 2 d | 3 y | CAVB | 2 | LCA | New cardiac murmur due to tricuspid regurgitation | Alive |
| 10 | 2017 | Japan3 | 6 d | 8 y | CAVB | 1 | PA, AV | CHF, SM | Alive 10 y |
| 11 | 2012 | Canada2 | No detailed information | No detailed information | No detailed information | No detailed information | No detailed information | No detailed information | No detailed information |
| 12 | 2012 | U.S.8 | <1 mo (Newborn) | 20 y | CAVB | 1 | LAD, LCX | Unresponsiveness, cyanosis, cardiac arrest | Death |
| 13 | 2015 | Canada4 | Unspecified age at PMI (EW implantation) and age at CS. Implantation-to-C interval: 3 y | CAVB | Unspecified | LV, RV (left and right ventricular walls) | Unspecified | Alive | |
| 14 | 2015 | Canada4 | Unspecified age at PMI (EW implantation) and age at CS. Implantation-to-C interval: 7 y | CAVB | Unspecified | LCX | Exercise-associated chest pain unresponsiveness | Death | |
| 15 | 2018 2017 | Slovak Republic9 | 14 d 29 y | CAVB (fetal myocarditis) | 1 | LCA, RCA | Shortness of breath, chest pain, and malaise | Death | |
AV = atrioventricular; CAVB = complete atrioventricular block; CHF = chronic heart failure; cJET = congenital junctional ectopic tachycardia; CS = cardiac strangulation; CTGA = corrected transposition of great arteries; LCA = left coronary artery; LCX = left circumflex artery; LV = left ventricle; PA = pulmonary artery; PMI = pacemaker implantation; RCA = right coronary artery; RVOT = right ventricular outflow tract; SM = systolic murmur; TOF = tetralogy of Fallot; VSD = ventricular septal defect.
Mah et al1 in 2018 found a higher incidence of coronary artery compression by epicardial leads (5.5%) than previously reported in the literature. They associated it with an increase in the number of publications about this complication.
Alhuzaimi et al5 think the redundant leads must not be looped very long anteriorly around the cardiac chambers nor placed inside the pericardium to prevent CS in neonates or even infants undergoing implantation of an epicardial pacing system. The authors reported that excess leads can be placed in the pleural space with the generator implanted on the diaphragmatic surface of the same pleural space. Chihiro et al3 suggest using an expanded polytetrafluoroethylene sheet to separate the heart and leads or circling tiny counterclockwise loop of lead. That might be an effective method of preventing CS.
The absence of symptoms does not exclude the possibility of CS formation, which is supported by the presented clinical examples. After the implantation, the chest x-ray only in the frontal view was performed. Lead pathology was not described.
Due to the experience of our first patient, we started to perform chest x-ray in the lateral view for all patients with pacemakers implanted at an early age. The second patient underwent chest x-ray in the lateral view at 2.6 years after the implantation. The lead loop around the heart was a random find. The excess lead loops probably slipped around the heart immediately after the surgery. However, the absence of lateral X-rays did not allow us to suspect a lead dislocation.
Despite the absence of coronary vessel compression signs and other heart structures according to multispiral computed tomography data, it is planned to extract the leads. A similar case of CS after epicardial pacemaker implantation also was observed in a patient at 8 years after implantation of a single-chamber pacemaker at the neonatal period. A retrospective analysis of x-rays showed that looping around the heart in this child was formed in the 14 days after pacemaker implantation.3 Management of cases without clinical symptoms and signs of heart compression remains unclear. Insufficient experience does not allow us to formulate a clear conclusion regarding the emergency of lead replacement in asymptomatic patients without hemodynamic disorders. Perhaps a wait-and-see tactic should be followed if the length of leads allows.
As we get more knowledge about the complications associated with epicardial leads, it is necessary to improve the surgical technique to avoid compression of the coronary arteries and heart structures.
Conclusions
Until the active growth of the child is completed, we should remember CS. Special attention should be paid to the children who underwent pacemaker implantation during the first year of life, due to more intense physical development and a high probability of the CS formation with excessive lead length.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the patients for allowing us to share his details.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Mah D.Y., Prakash A., Porras D., Fynn-Thompson F., DeWitt E.S., Banka P. Coronary artery compression from epicardial leads: more common than we think. Heart Rhythm. 2018;15:1439–1447. doi: 10.1016/j.hrthm.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi D., Tomizawa Y. Cardiac strangulation from epicardial pacemaker leads: diagnosis, treatment, and prevention. Gen Thorac Cardiovasc Surg. 2015;63:22–29. doi: 10.1007/s11748-014-0483-x. [DOI] [PubMed] [Google Scholar]
- 3.Chihiro M., Yoshie O., Yusuke A., et al. A case of cardiac strangulation following epicardial pacemaker implantation. Gen Thorac Cardiovasc Surg. 2020;68:1499–1502. doi: 10.1007/s11748-020-01337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carreras E.M., Duncan W.J., Djurdjev O., Campbell A.I. Cardiac strangulation following epicardial pacemaker implantation: a rare pediatric complication. J Thorac Cardiovasc Surg. 2015;149:522–527. doi: 10.1016/j.jtcvs.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 5.Alhuzaimi A., Roy N., Duncan W.J. Cardiac strangulation from epicardial pacemaker: early recognition and prevention. Cardiol Young. 2011;4:471–473. doi: 10.1017/S1047951111000242. [DOI] [PubMed] [Google Scholar]
- 6.Brenner J.I., Gaines S., Cordier J., Reiner B.I., Haney P.J., Gundry S.R. Cardiac strangulation: two-dimensional echo recognition of a rare complication of epicardial pacemaker therapy. Am J Cardiol. 1988;61:654–656. doi: 10.1016/0002-9149(88)90786-2. [DOI] [PubMed] [Google Scholar]
- 7.Salerno J.C., Johnston T.A., Chun T.U. Coronary compression by an epicardial pacing lead within the pericardium. J Cardiovasc Electrophysiol. 2007;18:786. doi: 10.1111/j.1540-8167.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S.B., Bartz P.J., Earing M.G., Sheil A., Nicolosi A., Woods R.K. Case report. Myocardial infarction due to a retained epicardial pacing wire. Ann Thorac Surg. 2012;94:1724–1726. doi: 10.1016/j.athoracsur.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 9.Janík M., Hejna P., Straka L., Krajcovic J., Novomesky F. Strangulation of the heart presenting as sudden cardiac death: a deadly but forgotten complication of epicardial pacing device. Leg Med. 2018;32:107–112. doi: 10.1016/j.legalmed.2018.04.001. [DOI] [PubMed] [Google Scholar]