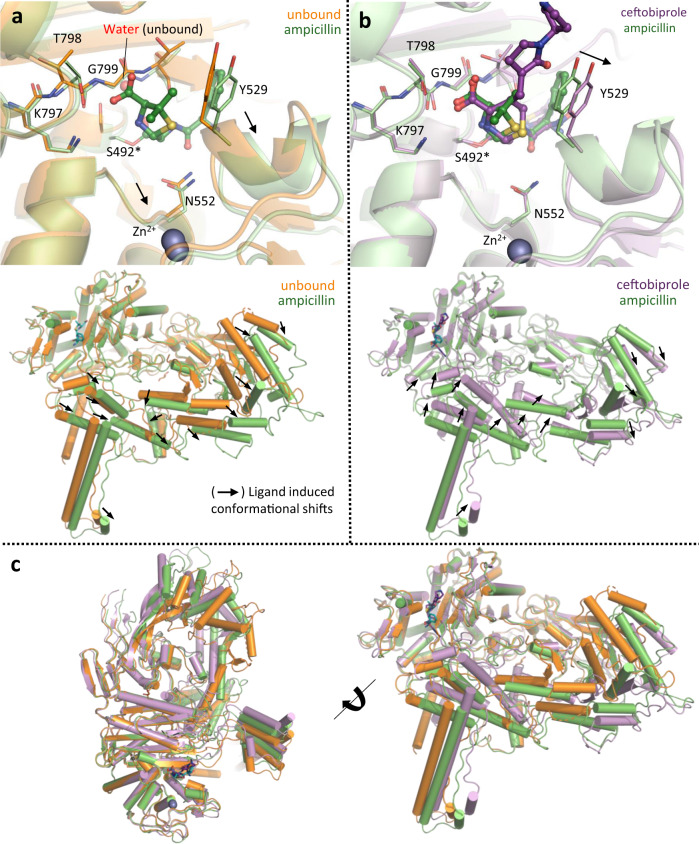
Fig. 3. β-lactam binding opens the active site of PBP2 and induces global conformational changes.
A superimposition of (a) unbound C. difficile PBP2 (orange) with ampicillin-bound form (green) shows β-lactam binding causes major conformational changes in the active site (top panel) and peripheral regions (bottom panel). Significant movements are indicated by arrows. b By comparing the complex structure of ampicillin (green) with ceftobiprole (purple) we find larger conformational changes in the active site (top panel) and overall (bottom panel) that are consistent with the larger size of the cephem core. c A superimposition of all three structures highlights the global conformation differences in all three structures, with movements mostly localized to the coiled-coil stretch, central-helix cluster, and NTD.