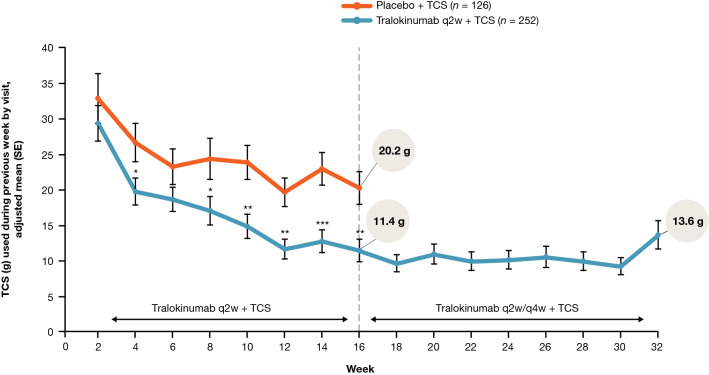
Fig. 6.
TCS use from baseline to Week 32 by visit. Assuming no TCS was used from non-returned tubes. Hypothetical estimand: treatments were reassigned at Week 16 and the placebo arm was only followed up to Week 16. The tralokinumab arm was followed beyond Week 16 and the different dosing (q2w or q4w) was ignored. Rescue medication was reset at Week 16. Data collected after permanent discontinuation of study medication or initiation of rescue medication were not included. In case of no post-baseline assessments before initiation of rescue medication, the Week 2 change was imputed as 0. Repeated-measurements model: Endpoint = Treatment*Week + Baseline*Week + Region + Baseline IGA. Compound symmetry was assumed for the covariance matrix. *p < 0.05 vs. placebo + TCS; **p < 0.01 vs. placebo + TCS; ***p < 0.001 vs. placebo + TCS. IGA Investigator’s Global Assessment, q2w every 2 weeks, q4w every 4 weeks, SE standard error, TCS topical corticosteroids