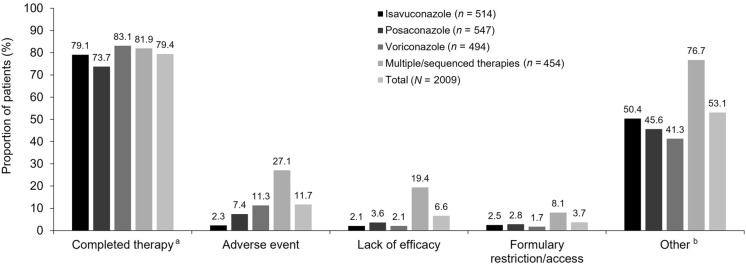
Fig. 2.
Reasons for discontinuation of mold-active triazoles (SAF). Patients were counted in multiple categories, but only once per category. Monotherapy was assigned to patients receiving one therapy throughout the study since index/enrollment. Percentages are based on number of patients with non-missing data for each category; isavuconazole n = 484, posaconazole n = 498, voriconazole n = 479, multiple/sequenced MAT therapies n = 454, and total N = 1915. aMultiple/sequenced MAT therapies described patients receiving more than one mold-active triazole therapy throughout the study since index/enrollment. b ‘Other’ reasons for discontinuation of mold-active triazoles included, but were not limited to, hospital visits, including hospital admission, discharge, and inpatient and outpatient switching. SAF safety analysis set