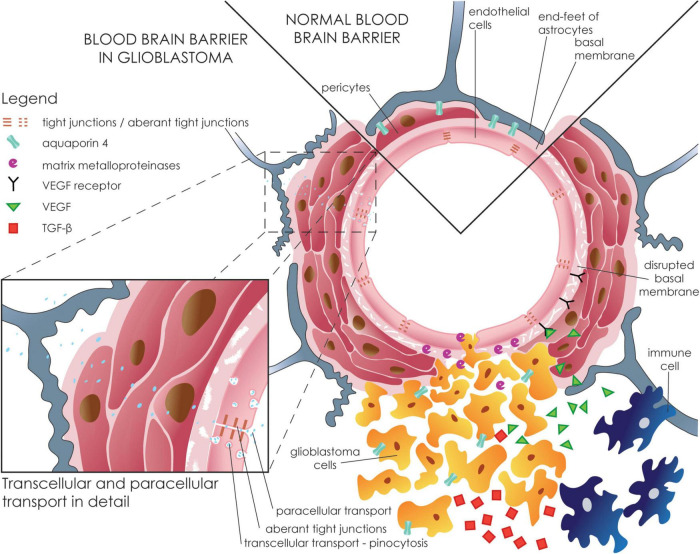
FIGURE 3.
Disruption of the blood-brain barrier in glioblastoma. GBM cells infiltrate the perivascular space with subsequent astrocytic end-feet displacement. Tumor, stromal, and immune cells occupy a specific tumor niche with a diverse proteomic profile but most importantly show upregulated VEGF and TGF-ß. VEGF binds to its receptor on endothelial cells leading to increased transendothelial permeability and downregulation of specific TJs (e.g., claudin-5, occludin, and ZO-1) and subsequent increased paracellular influx. Matrix metalloproteinases, most importantly MMP-9, that are secreted by tumor cells contribute to the disruption of the basal membrane by cleaving the ECM. Inset shows a more detailed view of the transcellular and paracellular movements across the BBB. The endothelium forms the inner layer of the BBB. GBM promotes a “leaky” phenotype in endothelial cells with increased transcellular transport and endothelial fenestrations. Additionally, down-regulation of TJs leads to increased paracellular transport with subsequent edema formation.